Abstract
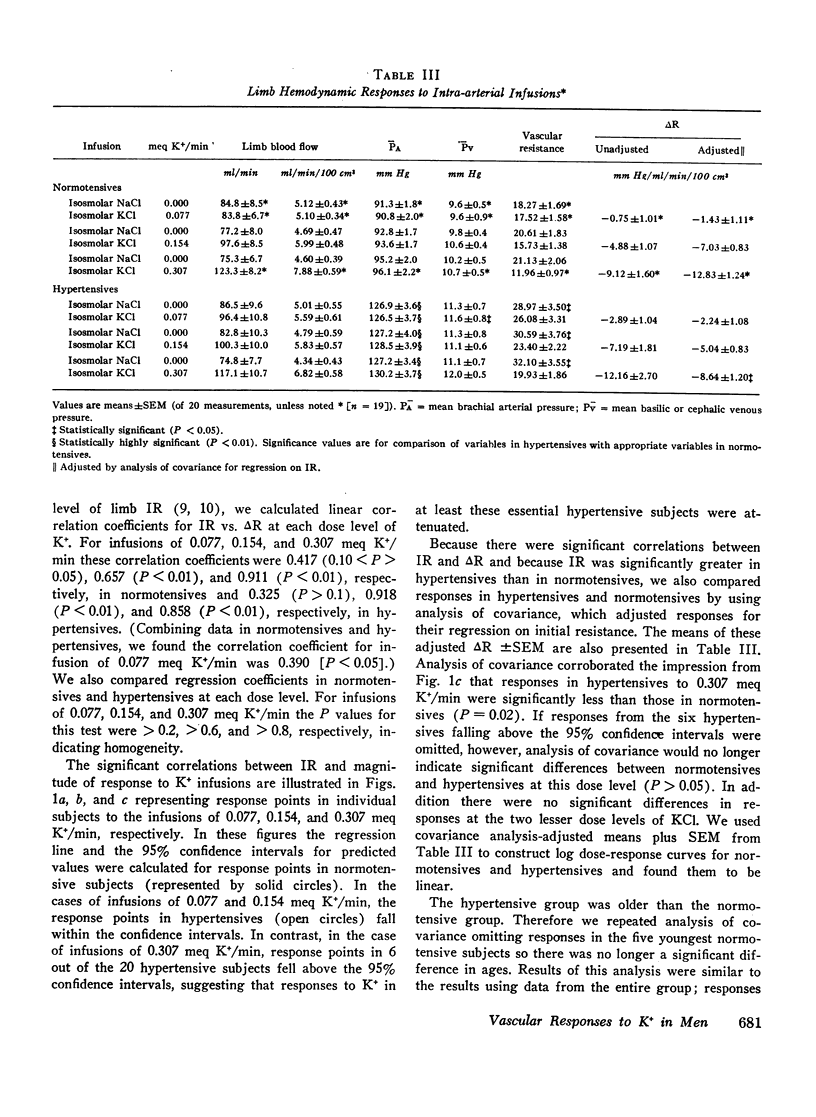
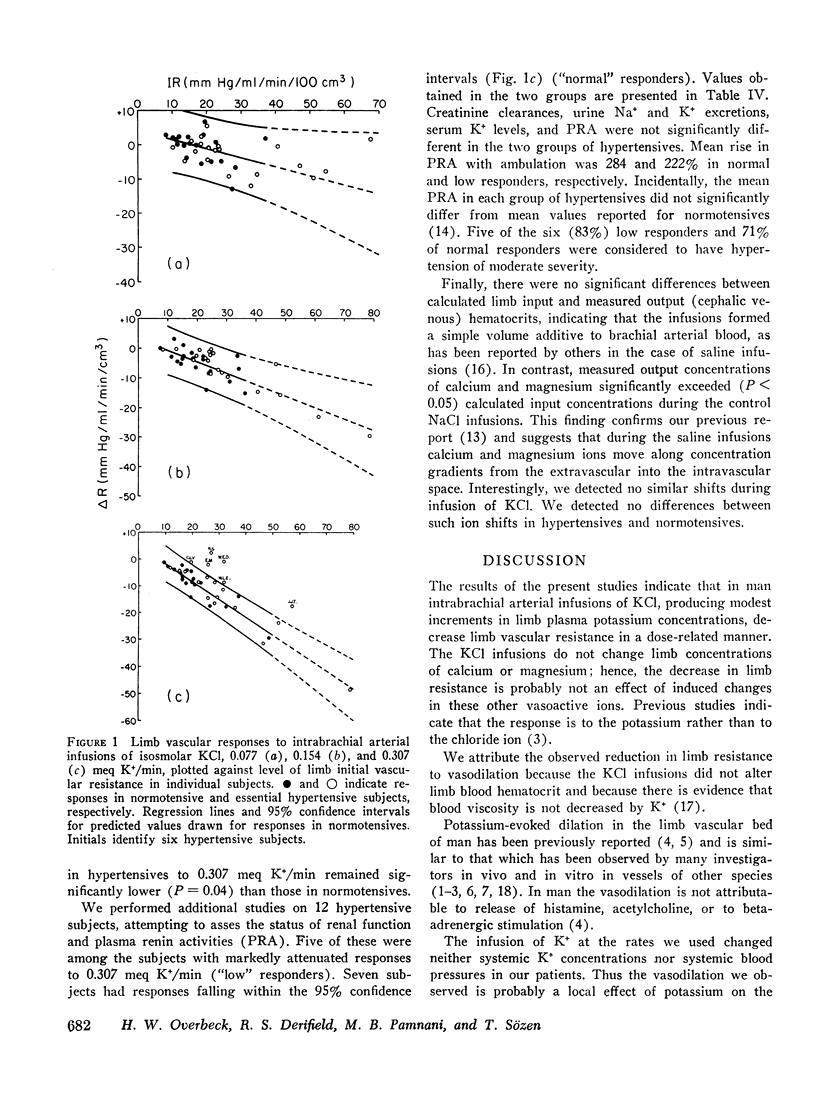
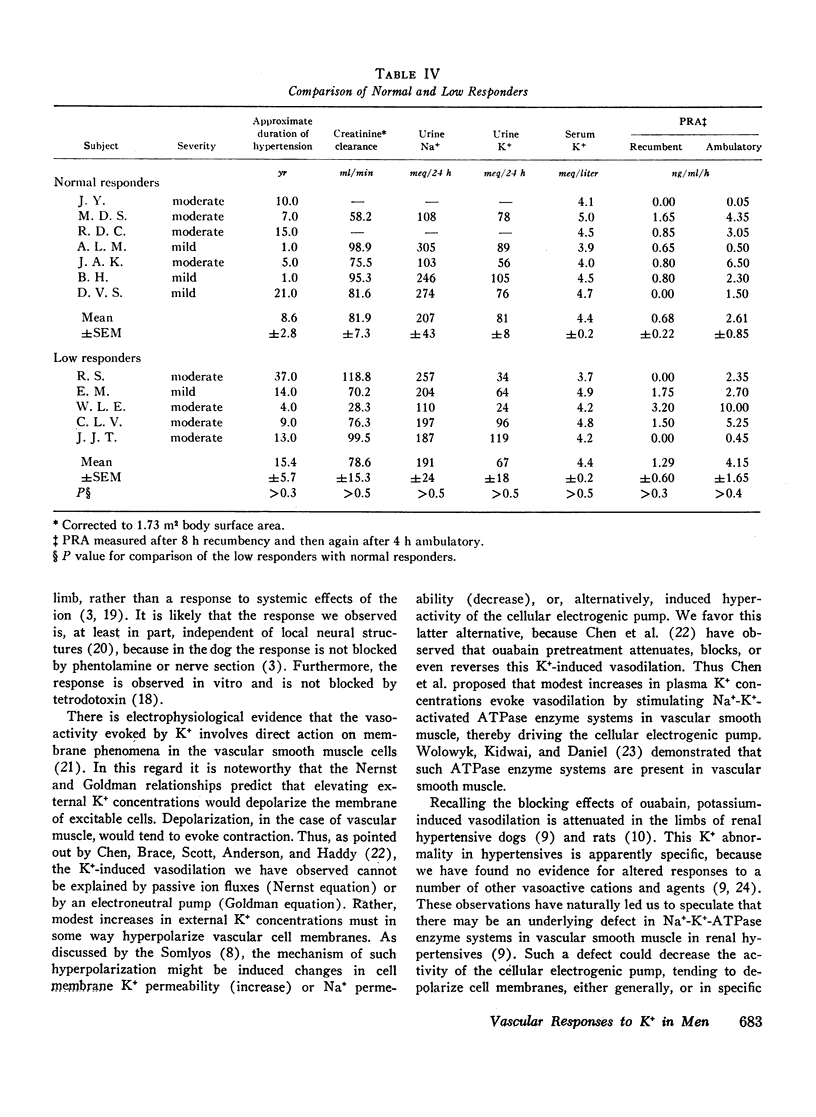
To study limb vascular responses to K+ in man, paired intrabrachial arterial infusions of isosmolar NaCl (control) and isosmolar KCl (0.077, 0.154, and 0.307 meq K+/min) in isosmolar NaCl were made in 20 normotensive men and 20 men with essential hypertension of mild to moderate severity. Limb blood pressures were monitored, limb blood flow was measured by indicator-dilution, and limb vascular resistance was calculated as mm Hg/ml flow/min/100 cm3 limb volume. Measured concentrations of K+ in limb venous plasma during infusion of 0.307 meq K+/min ranged from 4.8 to 9.0 meq/liter. Changes in limb venous hematocrit, sodium, calcium, magnesium, and osmolality were similar during control and KCl infusions. The infusions did not significantly change systemic blood K+ concentration or blood pressures. Compared to NaCl, KCl decreased limb resistance (P < 0.05) in both normotensives and hypertensives, in a dose-related manner. Resting limb vascular resistances (IR) in hypertensives were greater (P < 0.05) than those in normotensives. Despite a positive correlation (P < 0.05) between IR and magnitude of response to K+, responses in hypertensives to K+ were not greater than those in normotensives. Further, analysis of covariance indicated that responses to 0.307 meq K+/min in hypertensives as a group were, in fact, less (P = 0.02) than those in normotensives. These results indicate that the vasodilator response to K+ may be attenuated in a significant proportion of essential hypertensive men, as it is in renal hypertensive animals. These abnormal responses to K+ in hypertensives may indicate an underlying defect in vascular K+ metabolism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson J., Wahlström B., Johansson B., Jonsson O. Influence of the ionic environment on spontaneous electrical and mechanical activity of the rat portal vein. Circ Res. 1967 Nov;21(5):609–618. doi: 10.1161/01.res.21.5.609. [DOI] [PubMed] [Google Scholar]
- Bandick N. R., Sparks H. V. Contractile response of vascular smooth muscle of renal hypertensive rats. Am J Physiol. 1970 Aug;219(2):340–344. doi: 10.1152/ajplegacy.1970.219.2.340. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Brace R. A., Scott J. B., Anderson D. K., Haddy F. J. The mechanism of the vasodilator action of potassium. Proc Soc Exp Biol Med. 1972 Jul;140(3):820–824. doi: 10.3181/00379727-140-36560. [DOI] [PubMed] [Google Scholar]
- Dawes G. S. The vaso-dilator action of potassium. J Physiol. 1941 Jan 14;99(2):224–238. doi: 10.1113/jphysiol.1941.sp003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMANUEL D. A., SCOTT J. B., HADDY F. J. Effect of potassium on small and large blood vessels of the dog forelimb. Am J Physiol. 1959 Sep;197:637–642. doi: 10.1152/ajplegacy.1959.197.3.637. [DOI] [PubMed] [Google Scholar]
- GOLDBLATT H. Hypertension and vascular disease. Circulation. 1960 May;21:643–645. doi: 10.1161/01.cir.21.5.643. [DOI] [PubMed] [Google Scholar]
- HADDY F. J., OVERBECK H. W. Electrolytes--the common denominator in hypertension? J Ark Med Soc. 1962 Apr;58:477–481. [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Johansson B., Bohr D. F. Rhythmic activity in smooth muscle from small subcutaneous arteries. Am J Physiol. 1966 Apr;210(4):801–806. doi: 10.1152/ajplegacy.1966.210.4.801. [DOI] [PubMed] [Google Scholar]
- Konold P., Gebert G., Brecht K. The effect of potassium on the tone of isolated arteries. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(4):285–291. doi: 10.1007/BF00362638. [DOI] [PubMed] [Google Scholar]
- Minkoff L., Gaertner G., Darab M., Mercier C., Levin M. L. Inhibition of brain sodium-potassium ATPase in uremic rats. J Lab Clin Med. 1972 Jul;80(1):71–78. [PubMed] [Google Scholar]
- Overbeck H. W., Daugherty R. M., Jr, Haddy F. J. Continuous infusion indicator dilution measurement of limb blood flow and vascular response to magnesium sulfate in normotensive and hypotensive men. J Clin Invest. 1969 Oct;48(10):1944–1957. doi: 10.1172/JCI106161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck H. W., Grega G. J. Response of the limb vascular bed in man to intrabrachial arterial infusions of hypertonic dextrose or hypertonic sodium chloride solutions. Circ Res. 1970 Jun;26(6):717–731. doi: 10.1161/01.res.26.6.717. [DOI] [PubMed] [Google Scholar]
- Overbeck H. W. Vascular responses to cations, osmolality, and angiotensin in renal hypertensive dogs. Am J Physiol. 1972 Dec;223(6):1358–1364. doi: 10.1152/ajplegacy.1972.223.6.1358. [DOI] [PubMed] [Google Scholar]
- Scott J. B., Daugherty R. M., Jr, Overbeck H. W., Haddy F. J. Vascular effects of ions. Fed Proc. 1968 Nov-Dec;27(6):1403–1407. [PubMed] [Google Scholar]
- Sivertsson R. The hemodynamic importance of structural vascular changes in essential hypertension. Acta Physiol Scand Suppl. 1970;343:1–56. [PubMed] [Google Scholar]
- Skinner N. S., Jr, Costin J. C. Role of O2 and K+ in abolition of sympathetic vasoconstriction in dog skeletal muscle. Am J Physiol. 1969 Aug;217(2):438–444. doi: 10.1152/ajplegacy.1969.217.2.438. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Stein I. M., Perez G., Johnson R., Cummings N. B. Serum levels and urinary excretion of methylguanidine in chronic renal failure. J Lab Clin Med. 1971 Jun;77(6):1020–1024. [PubMed] [Google Scholar]
- TOBIAN L., JANECEK J., TOMBOULIAN A., FERREIRA D. Sodium and potassium in the walls of arterioles in experimental renal hypertension. J Clin Invest. 1961 Oct;40:1922–1925. doi: 10.1172/JCI104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J. A dye dilution method for the determination of brachial artery blood flow during forearm exercise in man. Acta Physiol Scand. 1965 Aug;64(4):477–478. doi: 10.1111/j.1748-1716.1965.tb04207.x. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Mierzwiak D. S., Skinner N. S., Jr, Mitchell J. H. Potassium-induced cardiovascular and ventilatory reflexes from the dog hindlimb. Am J Physiol. 1968 Sep;215(3):542–548. doi: 10.1152/ajplegacy.1968.215.3.542. [DOI] [PubMed] [Google Scholar]
- Wolowyk M. W., Kidwai A. M., Daniel E. E. Sodium-potassium stimulated adenosinetriphosphatase of vascular smooth muscle. Can J Biochem. 1971 Mar;49(3):376–384. doi: 10.1139/o71-055. [DOI] [PubMed] [Google Scholar]


