Abstract
During phagocytosis, new phospholipid is synthesized from triglyceride fatty acid and may be utilized to form the membranes of phagocytic vesicles. In addition, hydrogen peroxide, which can peroxidize unsaturated fatty acids, is generated. Since both of these processes could change membrane fatty acid composition during the conversion of cytoplasmic granules and plasma membranes to phagosomes, the lipid compositions of these structures were examined. Phagocytic vesicles were prepared by density gradient centrifugation of polystyrene latex particles after phagocytosis. Granule and plasma membrane fractions were isolated by density gradient and differential centrifugation. Phospholipids and fatty acids were analyzed by thin-layer chromatography and gas-liquid chromatography.
While whole cells, granules, plasma membranes, and phagosomes were all similar in phospholipid composition, phagosome fatty acids were significantly more saturated than those of the other fractions. This was primarily due to reduced oleic and arachidonic acids and increased palmitic acid in the phagocytic vesicle lipids. Plasma membrane was also more saturated in comparison to whole cells and granules. However, this difference was not sufficient to explain the marked comparative saturation of the phagosomes. The observed increase in fatty acid saturation in these lipids may have been induced by a combination of either peroxidative destruction of polyunsaturated fatty acids or phospholipase activity, coupled with reacylation mechanisms favoring saturated fatty acids.
Full text
PDF

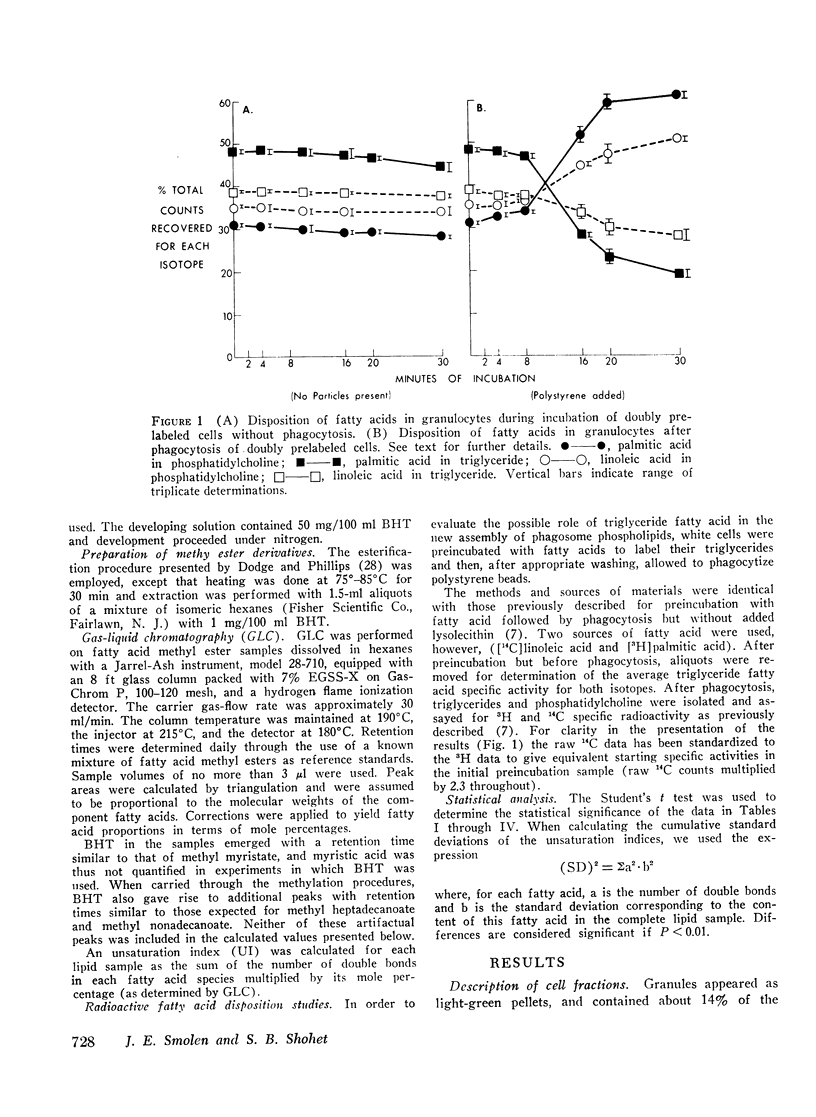

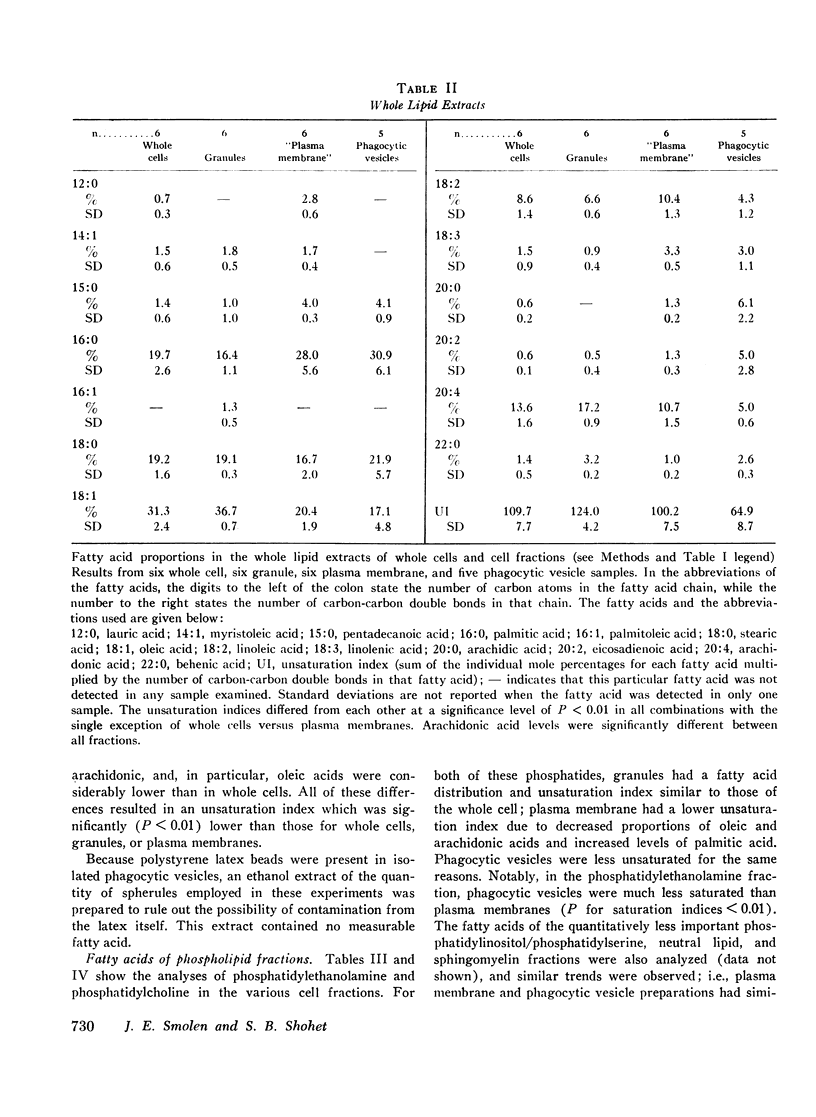
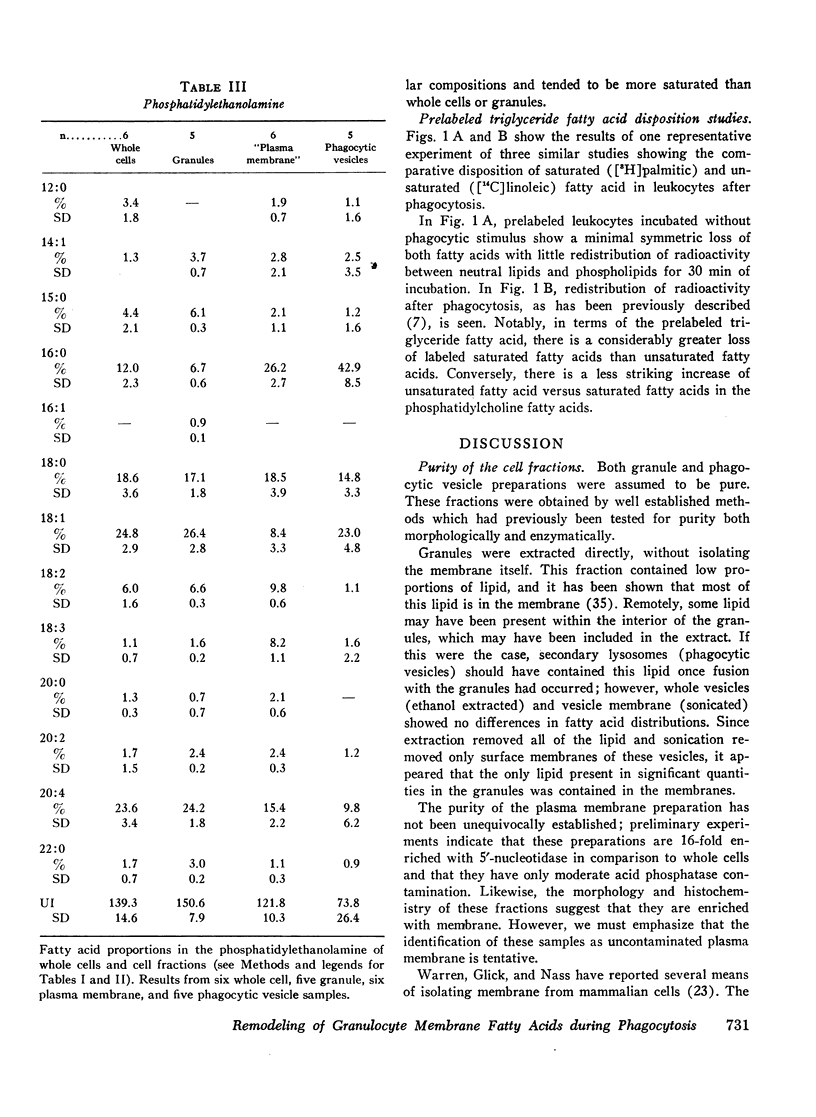
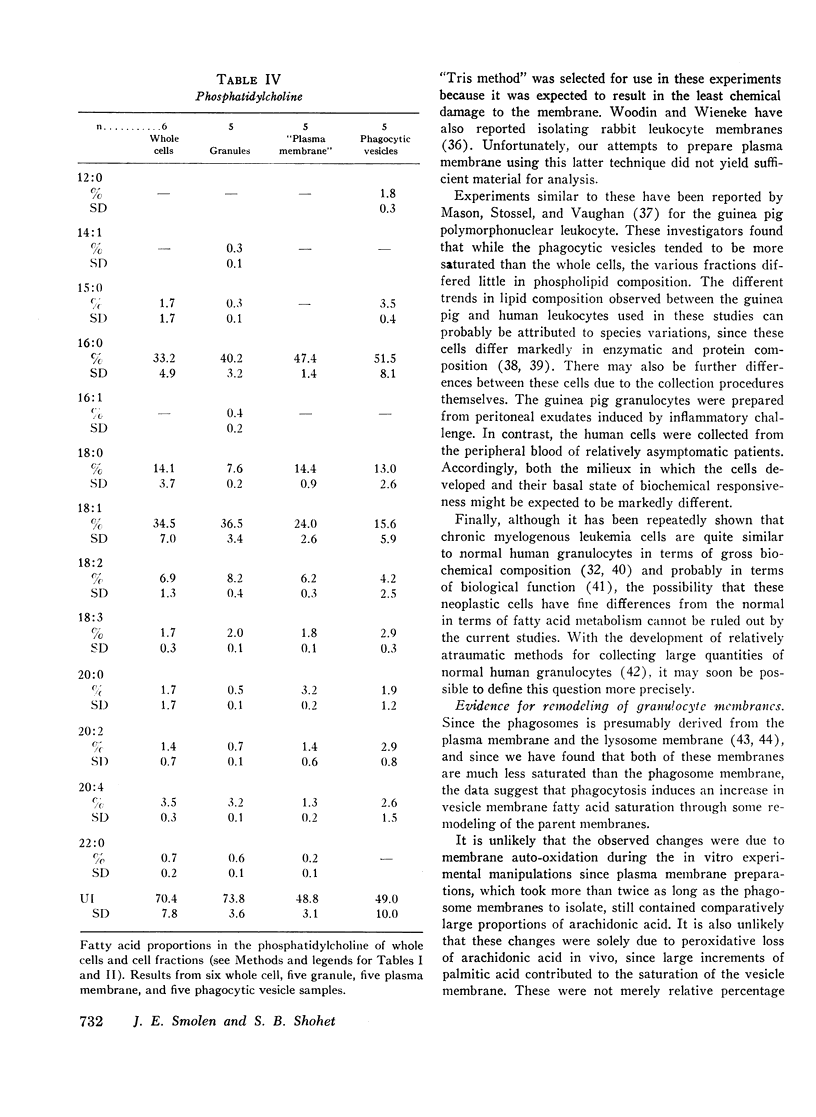


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff H., Hoyle R. J., Wolmark N. Positional distribution of fatty acids in triglycerides of animal depot fats. Biochim Biophys Acta. 1966 Feb 1;116(1):67–72. doi: 10.1016/0005-2760(66)90092-0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLE L. K., HILL E. G., HOLMAN R. T. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962 Aug;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- DESNUELLE P., SAVARY P. SPECIFICITIES OF LIPASES. J Lipid Res. 1963 Oct;4:369–384. [PubMed] [Google Scholar]
- Demel R. A., Kinsky S. C., Kinsky C. B., van Deenen L. L. Effects of temperature and cholesterol on the glucose permeability of liposomes prepared with natural and synthetic lecithins. Biochim Biophys Acta. 1968 Jun 11;150(4):655–665. doi: 10.1016/0005-2736(68)90055-2. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Autoxidation as a cause of altered lipid distribution in extracts from human red cells. J Lipid Res. 1966 May;7(3):387–395. [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J Lipid Res. 1967 Nov;8(6):667–675. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Farrow S. Cellular triglyceride as a source of fatty acid for lecithin synthesis during phagocytosis. Biochim Biophys Acta. 1969 Mar 4;176(2):438–441. doi: 10.1016/0005-2760(69)90208-2. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Levy S. Increased synthesis of phospholipid during phagocytosis. J Clin Invest. 1968 Oct;47(10):2217–2229. doi: 10.1172/JCI105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Phospholipid metabolism by phagocytic cells. I. A comparison of conversion of [32P]lysolecithin to lecithin and glycerylphosphorylcholine by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. Biochim Biophys Acta. 1966 Dec 7;125(3):510–524. [PubMed] [Google Scholar]
- Elsbach P., Zucker-Franklin D., Sansaricq C. Increased lecithin synthesis during phagocytosis by normal leukocytes and by leukocytes of a patient with chronic granulomatous disease. N Engl J Med. 1969 Jun 12;280(24):1319–1322. doi: 10.1056/NEJM196906122802403. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gottfried E. L. Lipids of human leukocytes: relation to celltype. J Lipid Res. 1967 Jul;8(4):321–327. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L., WALLACH D. F. The metabolic basis of phagocytosis. III. Incorporation of inorganic phosphate into various classes of phosphatides during phagocytosis. J Biol Chem. 1961 Jul;236:1895–1901. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lubin B. H., Shohet S. B., Nathan D. G. Changes in fatty acid metabolism after erythrocyte peroxidation: stimulation of a membrane repair process. J Clin Invest. 1972 Feb;51(2):338–344. doi: 10.1172/JCI106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUSHITA S., IBUKI F., AOKI A. CHEMICAL REACTIVITY OF THE NUCLEIC ACID BASES. I. ANTIOXIDATIVE ABILITY OF THE NUCLEIC ACIDS AND THEIR RELATED SUBSTANCES ON THE OXIDATION OF UNSATURATED FATTY ACIDS. Arch Biochem Biophys. 1963 Sep;102:446–451. doi: 10.1016/0003-9861(63)90253-4. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Stossel T. P., Vaughan M. Lipids of alveolar macrophages, polymorphonuclear leukocytes, and their phagocytic vesicles. J Clin Invest. 1972 Sep;51(9):2399–2407. doi: 10.1172/JCI107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van Deenen L. L. The effect of alterations in fatty acid composition and cholesterol content on the permeability of Mycoplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1970;219(1):245–247. doi: 10.1016/0005-2736(70)90083-0. [DOI] [PubMed] [Google Scholar]
- OSTROWSKI W., TSUGITA A. Purification of acid phosphomonoesterase from the human prostate gland. Arch Biochem Biophys. 1961 Jul;94:68–78. doi: 10.1016/0003-9861(61)90012-1. [DOI] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Sastry P. S., Hokin L. E. Studies on the role of phospholipids in phagocytosis. J Biol Chem. 1966 Jul 25;241(14):3354–3361. [PubMed] [Google Scholar]
- Schwandt P., Birk J., Ehrhart H. Phospholipidzusammensetzung in leukämischen Leukocyten. Klin Wochenschr. 1969 Jan 15;47(2):111–112. doi: 10.1007/BF01745780. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. Changes in fatty acid metabolism in human leukemic granulocytes during phagocytosis. J Lab Clin Med. 1970 Apr;75(4):659–672. [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinberg H. M., Barber A. A. Studies on vitamin E action: peroxidation inhibition in structural protein-lipid micelle complexes derived from rat liver microsomal membranes. J Nutr. 1970 Apr;100(4):413–418. doi: 10.1093/jn/100.4.413. [DOI] [PubMed] [Google Scholar]
- Trotter N. L. Electron-opaque, lipid-containing bodies in mouse liver at early intervals after partial hepatectomy and sham operation. J Cell Biol. 1965 Jun;25(3 Suppl):41–52. doi: 10.1083/jcb.25.3.41. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Wetzel M. G., Korn E. D. Phagocytosis of latex beads by Acahamoeba castellanii (Neff). 3. Isolation of the phagocytic vesicles and their membranes. J Cell Biol. 1969 Oct;43(1):90–104. doi: 10.1083/jcb.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J., Perry S., Knutsen T. A., Gart J. J. Cell cycl characteristics, maturation, and phagocytosis in vitro of blast cells from patients with chronic myelocytic leukemia. Blood. 1971 Aug;38(2):153–161. [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. Composition and properties of a cell-membrane fraction from the polymorphonuclear leucocyte. Biochem J. 1966 May;99(2):493–500. doi: 10.1042/bj0990493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]


