Abstract
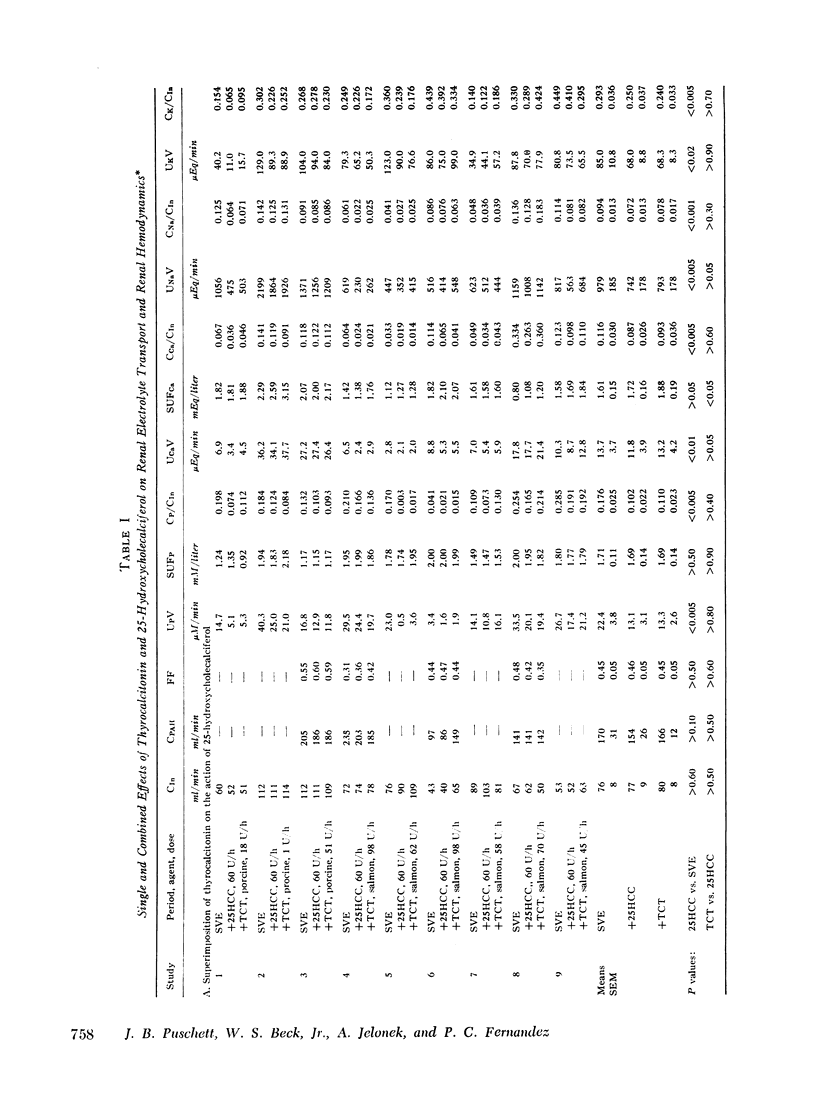
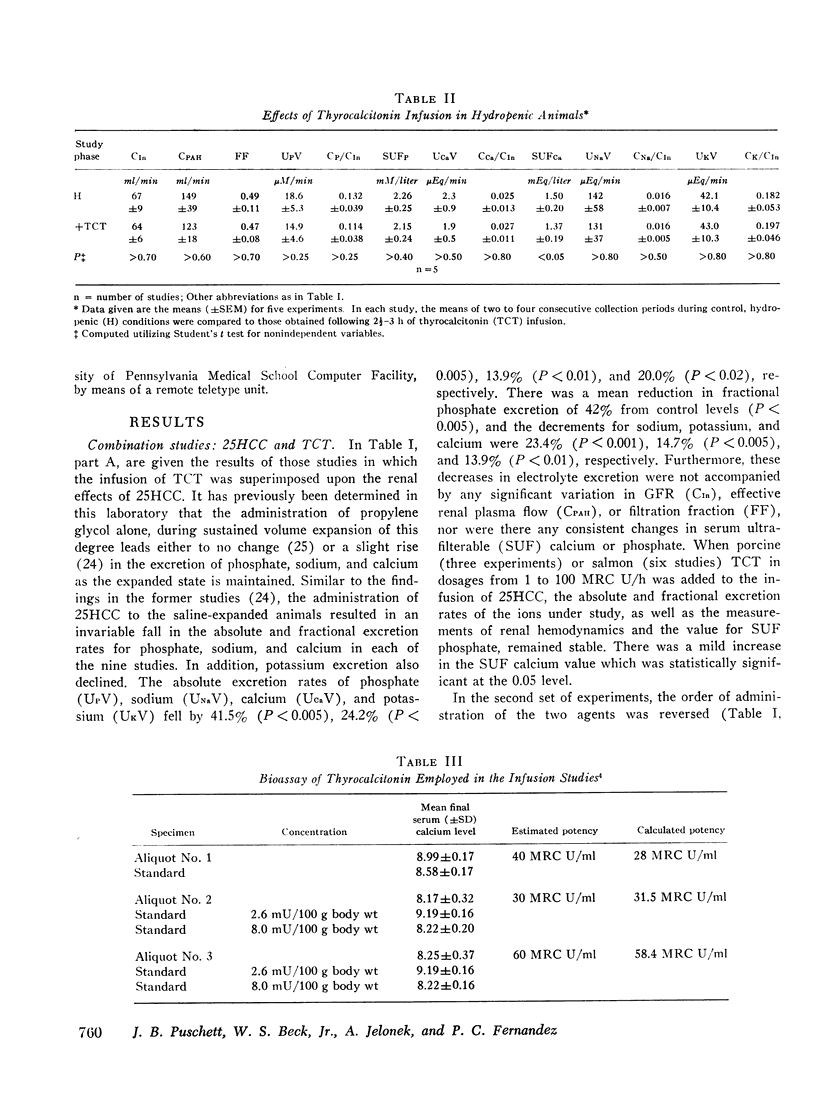
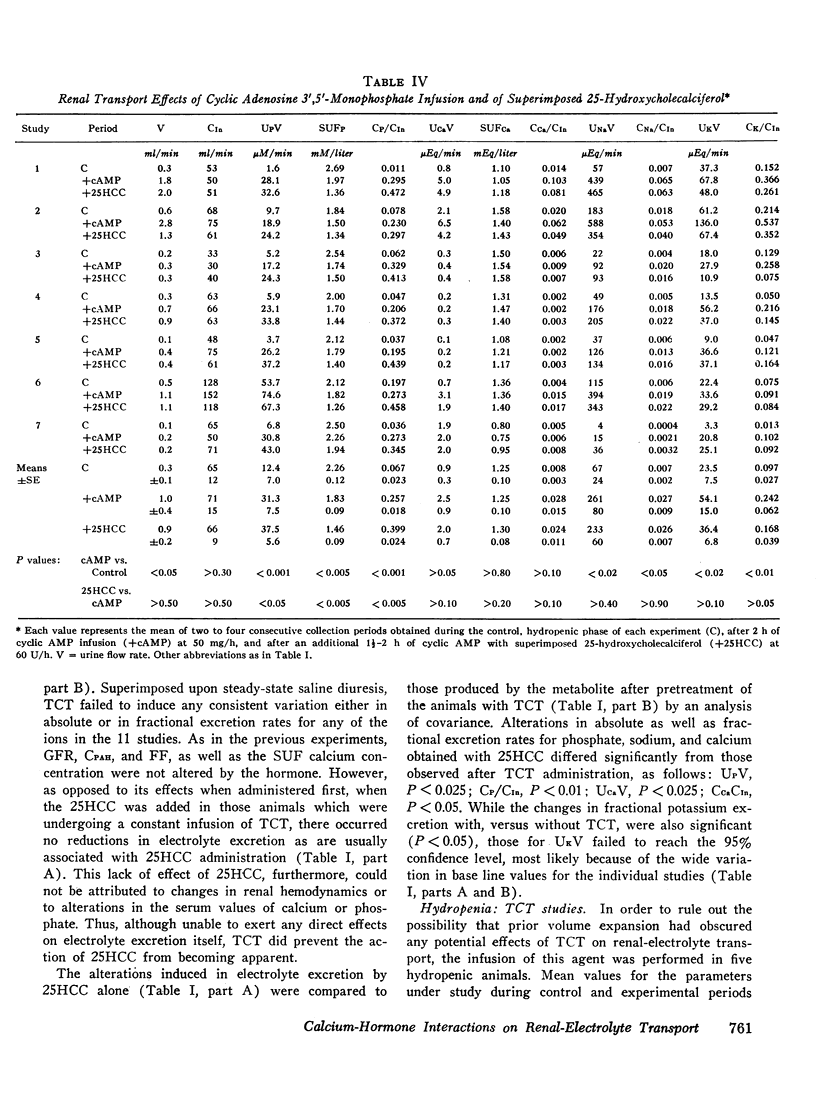
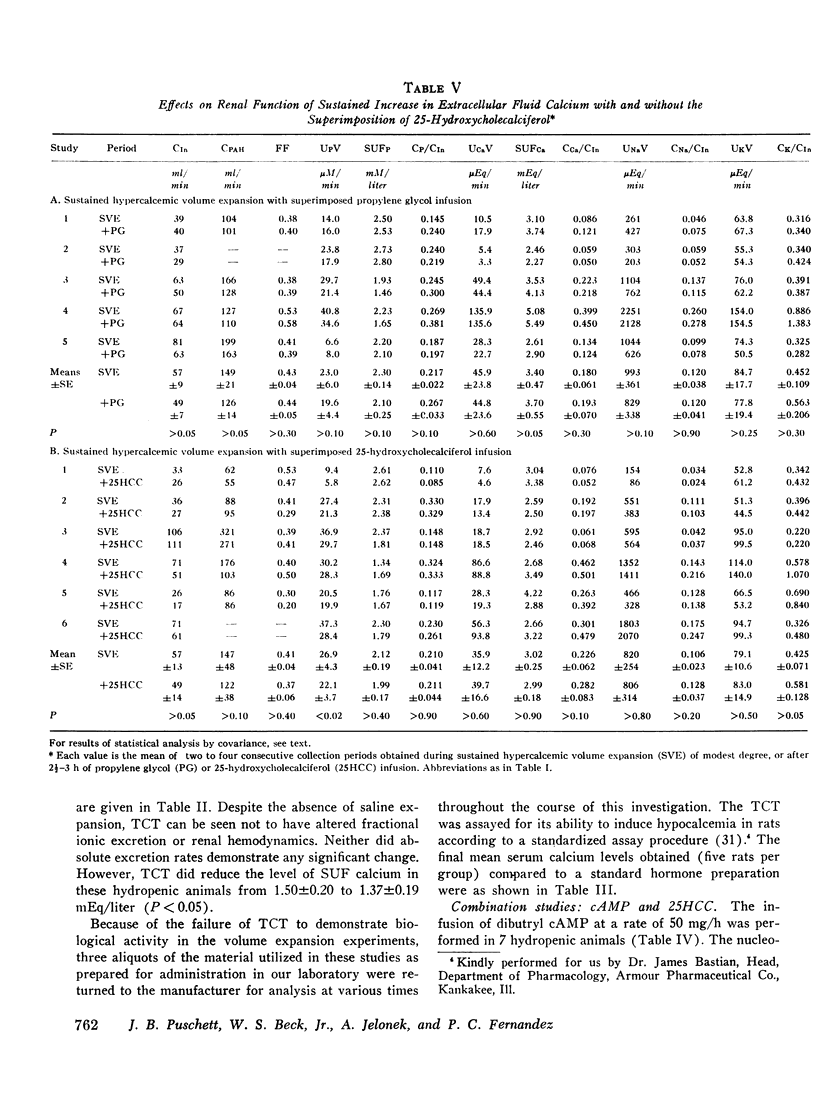
Acute clearance studies were performed in thyroparathyroidectomized animals to determine the actions and interactions of thyrocalcitonin (TCT), cyclic adenosine 3′5′-monophosphate (cAMP), 25-hydroxycholecalciferol (25HCC), and calcium ion on the reabsorption of phosphate, calcium, sodium, and potassium by the kidney. The infusion of 25HCC in a dosage of 60 U/h to moderately saline-expanded animals (2.5% body weight) induced a fall in the excretion of all of the ions under study after 90-120 min similar to that observed in previous experiments from this laboratory. Mean decrements in fractional excretion were: phosphate, 42.0% (P < 0.005); calcium, 25.0% (P < 0.005); sodium, 23.4% (P < 0.001); and potassium, 14.7% (P < 0.005). The superimposition of either porcine or salmon TCT (1-100 MRC U/h for 2 h) resulted in no further alterations in electrolyte excretion. However, the infusion of TCT during steady-state saline expansion, before the administration of 25HCC, obviated the renal transport effects of the vitamin D metabolite. Both in the latter studies, as well as those in which similar doses of TCT were given to hydropenic animals, the hormone itself failed to induce any consistent alteration in electrolyte excretion. Cyclic AMP (50 mg/h) caused an increase in the excretion of phosphate, sodium, and potassium and no change in calcium excretion. Like TCT, the nucleotide blocked the action of 25HCC on the kidney. Raising the mean level of serum ultrafilterable calcium to 3.02±0.25 mEq/liter from 1.62±0.17 mEq/liter likewise prevented enhanced ionic reabsorption due to 25HCC.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Gardner L. B., Beck L. H., Goldberg M. Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am J Physiol. 1973 May;224(5):1143–1148. doi: 10.1152/ajplegacy.1973.224.5.1143. [DOI] [PubMed] [Google Scholar]
- Agus Z. S., Puschett J. B., Senesky D., Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3',5'-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971 Mar;50(3):617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldred J. P., Kleszynski R. R., Bastian J. W. Effects of acute administration of porcine and salmon calcitonin on urine electrolyte excretion in rats. Proc Soc Exp Biol Med. 1970 Sep;134(4):1175–1180. doi: 10.3181/00379727-134-34968. [DOI] [PubMed] [Google Scholar]
- Ardaillou R., Fillastre J. P., Milhaud G., Rousselet F., Delaunay F., Richet G. Renal excretion of phosphate, calcium and sodium during and after a prolonged thyrocalcitonin infusion in man. Proc Soc Exp Biol Med. 1969 May;131(1):56–60. doi: 10.3181/00379727-131-33803. [DOI] [PubMed] [Google Scholar]
- Ardaillou R., Vuagnat P., Milhaud G., Richet G. Effets de la thyrocalcitonine sur l'excrétion rénale des phosphates, du calcium et des ions H+ chez l'homme. Nephron. 1967;4(5):298–314. doi: 10.1159/000179589. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D., Jr, Tenenhouse A. M., Rasmussen H. Parathyroid hormone. Annu Rev Physiol. 1967;29:349–372. doi: 10.1146/annurev.ph.29.030167.002025. [DOI] [PubMed] [Google Scholar]
- Bijvoet O. L., van der Sluys Veer J., de Vries H. R., van Koppen A. T. Natriuretic effect of calcitonin in man. N Engl J Med. 1971 Apr 1;284(13):681–688. doi: 10.1056/NEJM197104012841301. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Jr, Gilbert H. R., Avioli L. V. Intestinal calcium transport: the role of sodium. Science. 1972 Apr 14;176(4031):168–170. doi: 10.1126/science.176.4031.168. [DOI] [PubMed] [Google Scholar]
- Blythe W. B., Gitelman H. J., Welt L. G. Effect of expansion of the extracellular space on the rate of urinary excretion of calcium. Am J Physiol. 1968 Jan;214(1):52–57. doi: 10.1152/ajplegacy.1968.214.1.52. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Effects of purified parathyroid hormone on the calcium metabolism of monkey kidney cells. Endocrinology. 1968 Dec;83(6):1316–1322. doi: 10.1210/endo-83-6-1316. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Effects of thyrocalcitonin on calcium transport in kidney cells. Endocrinology. 1969 Aug;85(2):194–199. doi: 10.1210/endo-85-2-194. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in cell cultures. 3. Effects of calcium and parathyroid hormone in kidney cells. J Gen Physiol. 1970 Feb;55(2):163–186. doi: 10.1085/jgp.55.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Membrane transfer of calcium. Clin Orthop Relat Res. 1967 May-Jun;52:267–291. doi: 10.1097/00003086-196700520-00022. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Renal adenyl cyclase: anatomically separate sites for parathyroid hormone and vasopressin. Science. 1968 Feb 2;159(3814):545–547. doi: 10.1126/science.159.3814.545. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Kenny A. D. Hog thyrocalcitonin in the dog: urinary calcium, phosphorus, magnesium and sodium responses. Endocrinology. 1969 May;84(5):1199–1205. doi: 10.1210/endo-84-5-1199. [DOI] [PubMed] [Google Scholar]
- Frick A. Mechanism of inorganic phosphate diuresis secondary to saline infusions in the rat. Excretion of sodium, inorganic phosphate, and calcium in normal and in parathyroidectomized rats. Pflugers Arch. 1969;313(2):106–122. doi: 10.1007/BF00586239. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. R., Jr, Casper A. G. Renal effects of adenosine 3',5'-cyclic monophosphate and dibutyryl adenosine 3',5'-cyclic monophosphate. Evidence for a role for adenosine 3',5'-cyclic monophosphate in the regulation of proximal tubular sodium reabsorption. J Clin Invest. 1971 Jun;50(6):1231–1240. doi: 10.1172/JCI106600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. VITAMIN D AND PERMEABILITY OF INTESTINAL MUCOSA TO CALCIUM. Am J Physiol. 1965 Feb;208:370–374. doi: 10.1152/ajplegacy.1965.208.2.370. [DOI] [PubMed] [Google Scholar]
- Kaminsky N. I., Broadus A. E., Hardman J. G., Jones D. J., Jr, Ball J. H., Sutherland E. W., Liddle G. W. Effects of parathyroid hormone on plasma and urinary adenosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2387–2395. doi: 10.1172/JCI106458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler R., Walker V., Copp D. H. Natriuretic and diuretic effects of salmon calcitonin in rats. Can J Physiol Pharmacol. 1970 Dec;48(12):838–841. doi: 10.1139/y70-122. [DOI] [PubMed] [Google Scholar]
- Kenny A. D., Heiskell C. A. Effect of crude thyrocalcitonin on calcium and phosphorus metabolism in rats. Proc Soc Exp Biol Med. 1965 Oct;120(1):269–271. doi: 10.3181/00379727-120-30508. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M., Eknoyan G., Suki W. N. Natriuretic effects of vasopressin and cyclic AMP: possible site of action in the nephron. Am J Physiol. 1971 Jun;220(6):2013–2020. doi: 10.1152/ajplegacy.1971.220.6.2013. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Chapman L. W., Kleeman C. R. Effect of NaCl infusion on urinary Ca++ and Mg++ during reduction in their filtered loads. Am J Physiol. 1967 Nov;213(5):1218–1224. doi: 10.1152/ajplegacy.1967.213.5.1218. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. The influence of extracellular volume expansion on renal phosphate reabsorption in the dog. J Clin Invest. 1969 Jul;48(7):1237–1245. doi: 10.1172/JCI106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melson G. L., Chase L. R., Aurbach G. D. Parathyroid hormone-sensitive adenyl cyclase in isolated renal tubules. Endocrinology. 1970 Mar;86(3):511–518. doi: 10.1210/endo-86-3-511. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Morel F., Le Grimellec C. Phosphate, calcium and magnesium transfers in proximal tubules and loops of Henle, as measured by single nephron microperfusion experiments in the rat. Pflugers Arch. 1972;333(1):1–16. doi: 10.1007/BF00586037. [DOI] [PubMed] [Google Scholar]
- Nagata N., Rasmussen H. Parathyroid hormone, 3'5' AMP, Ca++, and renal gluconeogenesis. Proc Natl Acad Sci U S A. 1970 Feb;65(2):368–374. doi: 10.1073/pnas.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. B., Jr, Deluca H. F., Potts J. T., Jr Calcitonin inhibition of vitamin D-induced intestinal calcium absorption. Endocrinology. 1972 Jan;90(1):151–157. doi: 10.1210/endo-90-1-151. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Ruskin B., Casper A. Renal effects of porcine thyrocalcitonin in the dog. Endocrinology. 1970 Aug;87(2):262–270. doi: 10.1210/endo-87-2-262. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Agus Z. S., Senesky D., Goldberg M. Effects of saline loading and aortic obstruction on proximal phosphate transport. Am J Physiol. 1972 Oct;223(4):851–857. doi: 10.1152/ajplegacy.1972.223.4.851. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Fernandez P. C., Boyle I. T., Gray R. W., Omdahl J. L., DeLuca H. F. The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1972 Oct;141(1):379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Moranz J., Kurnick W. S. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972 Feb;51(2):373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Anast C., Arnaud C. Thyrocalcitonin, EGTA, and urinary electrolyte excretion. J Clin Invest. 1967 May;46(5):746–752. doi: 10.1172/JCI105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Ionic and hormonal control of calcium homeostasis. Am J Med. 1971 May;50(5):567–588. doi: 10.1016/0002-9343(71)90113-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Nagata N. Renal gluconeogenesis: effects of parathyroid hormone and dibutyryl 3',5'-AMP. Biochim Biophys Acta. 1970 Jul 21;215(1):17–28. doi: 10.1016/0304-4165(70)90383-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Pechet M., Fast D. Effect of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline, and other nucleotides upon calcium and phosphate metabolism. J Clin Invest. 1968 Aug;47(8):1843–1850. doi: 10.1172/JCI105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. J., Martin T. J., MacIntyre I. Phosphaturic effect of thyrocalcitonin. Lancet. 1966 Jul 9;2(7454):83–84. doi: 10.1016/s0140-6736(66)91807-1. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Casey P. A., Fleisch H. Simulation of phosphate excretion by the renal arterial infusion of 3'5'-AMP (cyclic AMP)-a possible mechanism of action of parathyroid hormone. Calcif Tissue Res. 1968;(Suppl):54–54a. doi: 10.1007/BF02065236. [DOI] [PubMed] [Google Scholar]
- STRICKLER J. C., THOMPSON D. D., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF INORGANIC PHOSPHATE EXCRETION IN THE RAT. J Clin Invest. 1964 Aug;43:1596–1607. doi: 10.1172/JCI105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands H., Kessler R. H. A calcium binding component of dog kidney cortex and its relationship to calcium transport. Proc Soc Exp Biol Med. 1971 Sep;137(4):1267–1273. doi: 10.3181/00379727-137-35770. [DOI] [PubMed] [Google Scholar]
- Schlueter R. J., Aldred J. P., Fisher J. D. Thyrocalcitonin: bioassay characteristics of purified preparations. Acta Endocrinol (Copenh) 1968 Jun;58(2):268–276. doi: 10.1530/acta.0.0580268. [DOI] [PubMed] [Google Scholar]
- Singer F. R., Woodhouse N. J., Parkinson D. K., Joplin G. F. Some acute effects of administered porcine calcitonin in man. Clin Sci. 1969 Aug;37(1):181–190. [PubMed] [Google Scholar]
- Staum B. B., Hamburger R. J., Goldberg M. Tracer microinjection study of renal tubular phosphate reabsorption in the rat. J Clin Invest. 1972 Sep;51(9):2271–2276. doi: 10.1172/JCI107036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H. Increased urinary phosphate excretion following volume expansion in normal man. Metabolism. 1970 Feb;19(2):129–139. [PubMed] [Google Scholar]
- Streeto J. M. Renal cortical adenyl cyclase: effect of parathyroid hormone and calcium. Metabolism. 1969 Nov;18(11):968–973. doi: 10.1016/0026-0495(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Martinez-Maldonado M., Rouse D., Terry A. Effect of expansion of extracellular fluid volume on renal phosphate handling. J Clin Invest. 1969 Oct;48(10):1888–1894. doi: 10.1172/JCI106155. [DOI] [PMC free article] [PubMed] [Google Scholar]


