Abstract
Hemolytic anemia caused by oxidative drugs is thought to result from the oxidation of intracellular and membrane sulfhydryl groups of the erythrocyte. This process is more likely to occur in those erythrocytes in which the intracellular mechanism for reduction of disulfides is abnormal (e.g., glucose-6-phosphate dehydrogenase deficiency). If a membrane sulfhydryl group is critical in the pathogenesis of druginduced hemolytic anemia, it follows that this specific group must be dependent on intracellular reductive mechanisms for maintenance of the reduced state.
This report describes a sulfhydryl group(s), involved in membrane structure, which is (are) dependent on intracellular metabolism for maintenance of the reduced state. It is postulated that this metabolically dependent membrane sulfhydryl group may play a role in the pathogenesis of drug-induced hemolytic anemia.
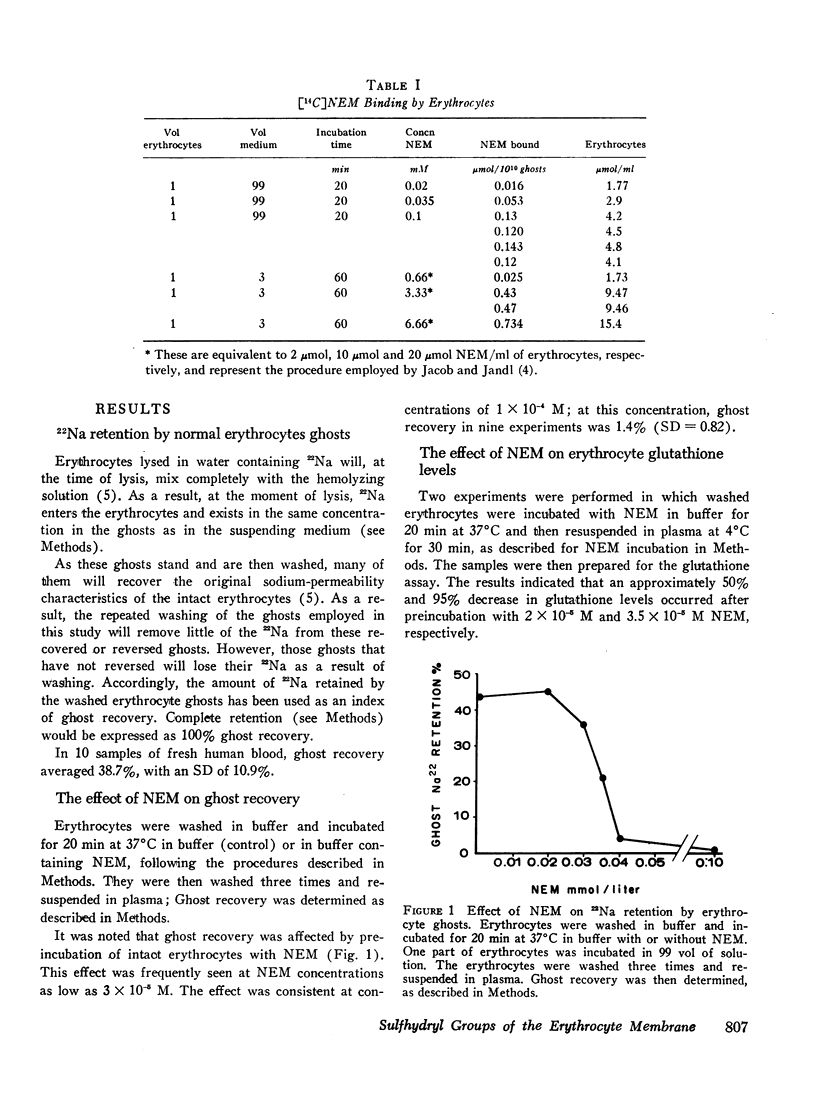
Membrane sulfhydryl groups were studied by observing the effect of sulfhydryl blocking agents, e.g., N-ethyl-maleimide (NEM), on the recovery of erythrocyte ghosts after osmotic lysis. It was shown that NEM interfered with ghost recovery by reacting with membrane sulfhydryl groups. The concentration of NEM (as determined by [14C] NEM binding) necessary to cause this effect was lower than that necessary to produce changes in osmotic fragility or cation permeability, or to cause Heinz body formation.
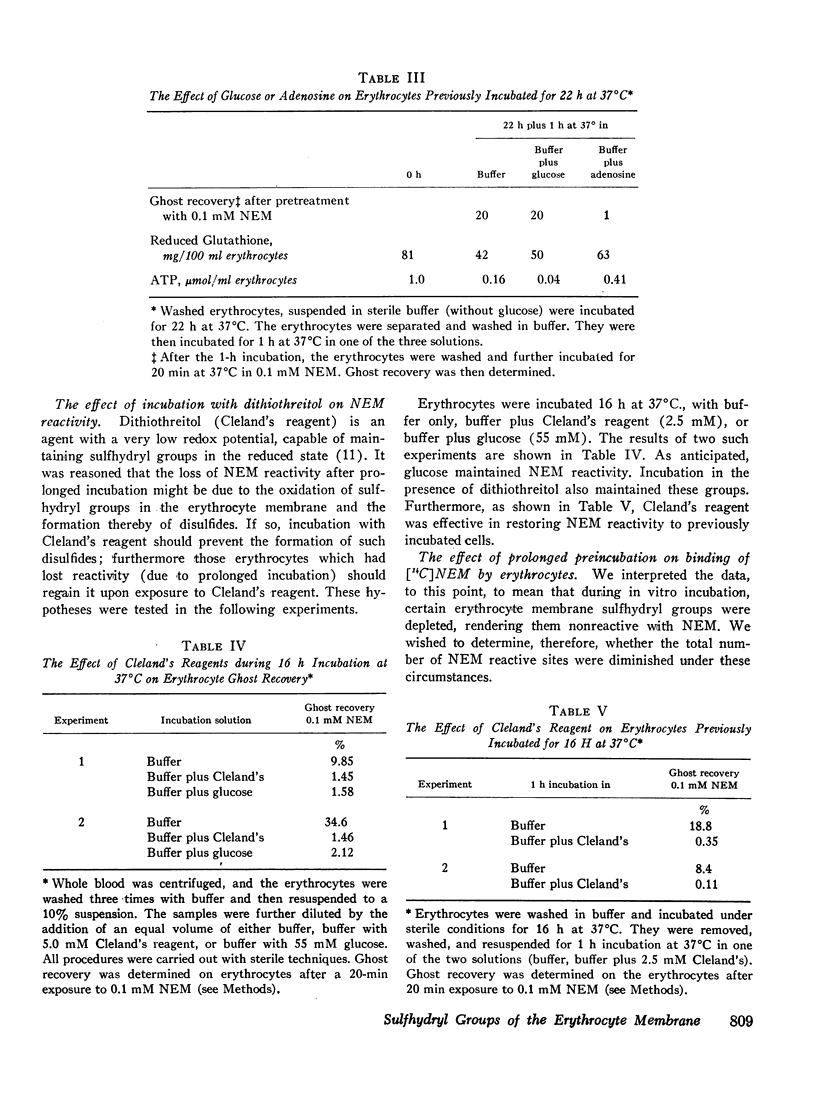
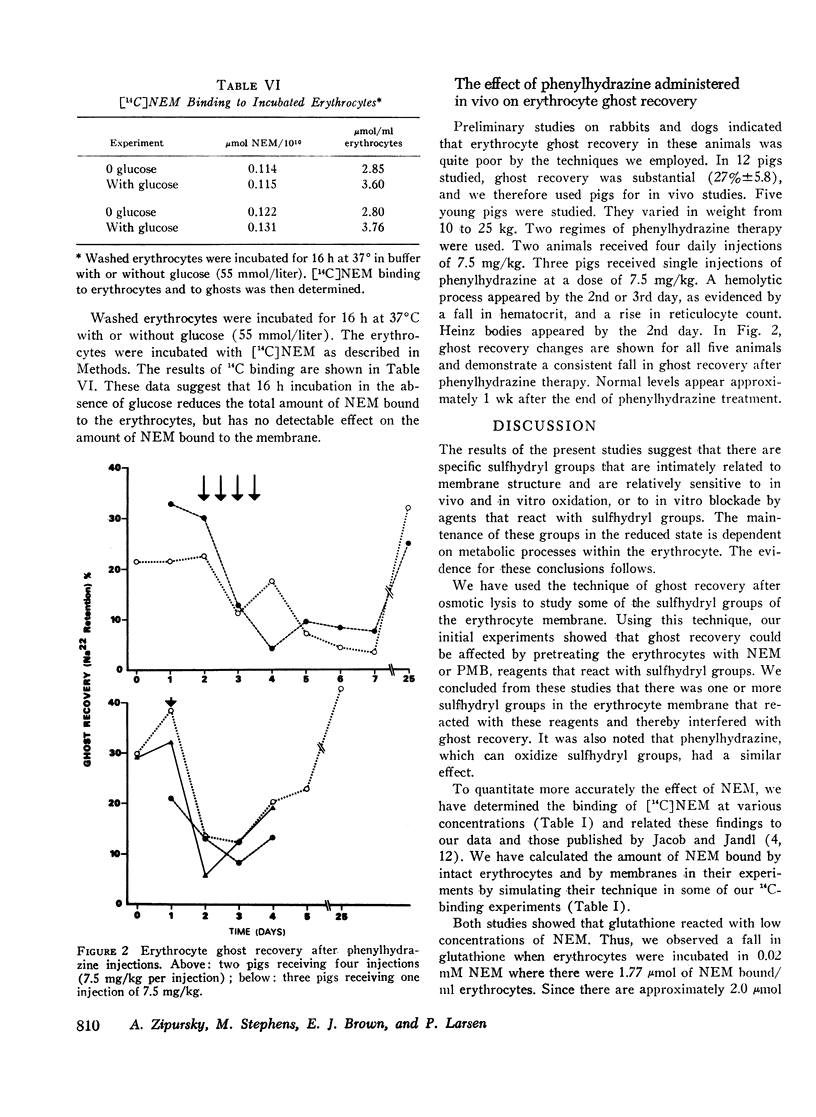
In the absence of glucose, these sulfhydryl groups became disulfides, but could be returned to the reduced state by restoring glycolysis or by adding dithiothreitol. Phenylhydrazine hemolytic anemia was induced in pigs, and membrane changes of the type described above occurred early in the pathogenesis of the disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969 Mar;21(1):73–103. [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER J. C., HOFFMAN J. F. FAILURE TO FIND INCREASED SODIUM, POTASSIUM-ATPASE IN RED CELL GHOSTS OF SCHIZOPHRENICS. Nature. 1964 Feb 22;201:823–823. doi: 10.1038/201823a0. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- SZEINBERG A., CLEJAN L. SULFHYDRYL GROUPS IN THE RED CELLS OF NORMAL AND GLUCOSE-6-PHOSPHATE DEHYDROGENASE-DEFICIENT SUBJECTS. Biochim Biophys Acta. 1964 Dec 9;93:564–572. doi: 10.1016/0304-4165(64)90340-x. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Permeability of normal and glucose-6-phosphate dehydrogenase deficient erythrocytes to glutathione. Biochem Biophys Res Commun. 1967 Sep 7;28(5):659–664. doi: 10.1016/0006-291x(67)90365-8. [DOI] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


