Abstract
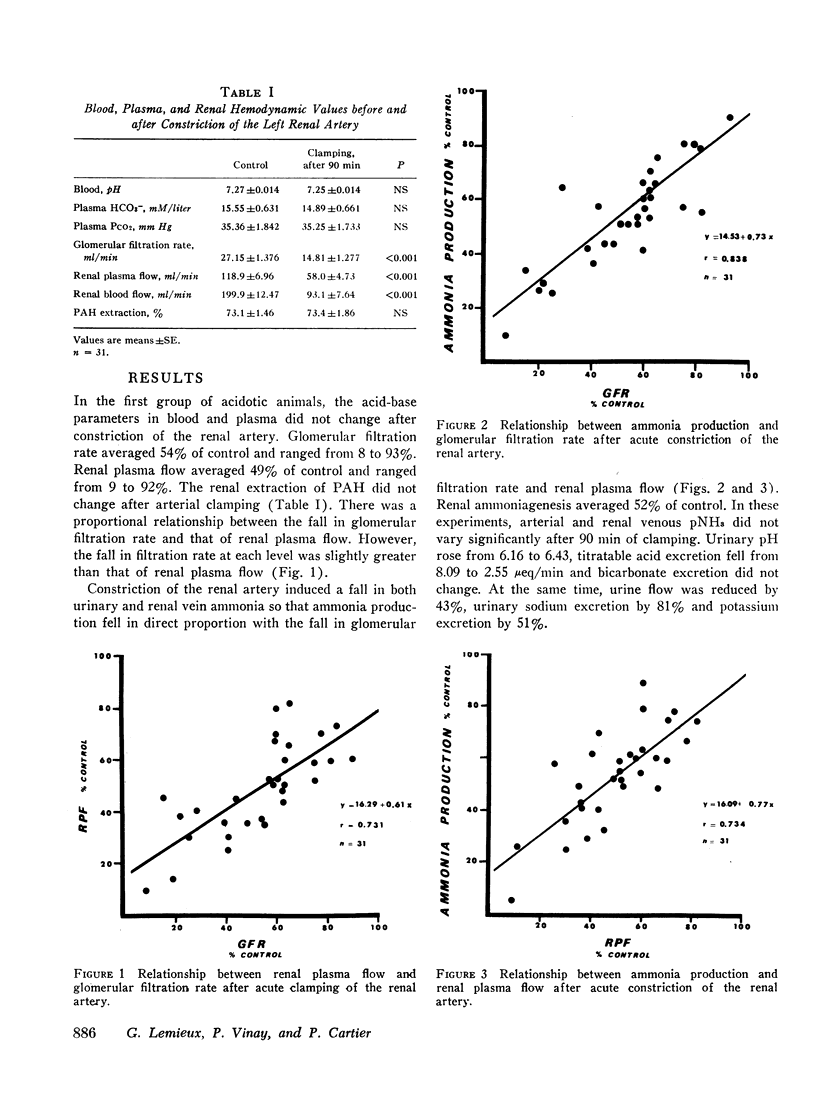
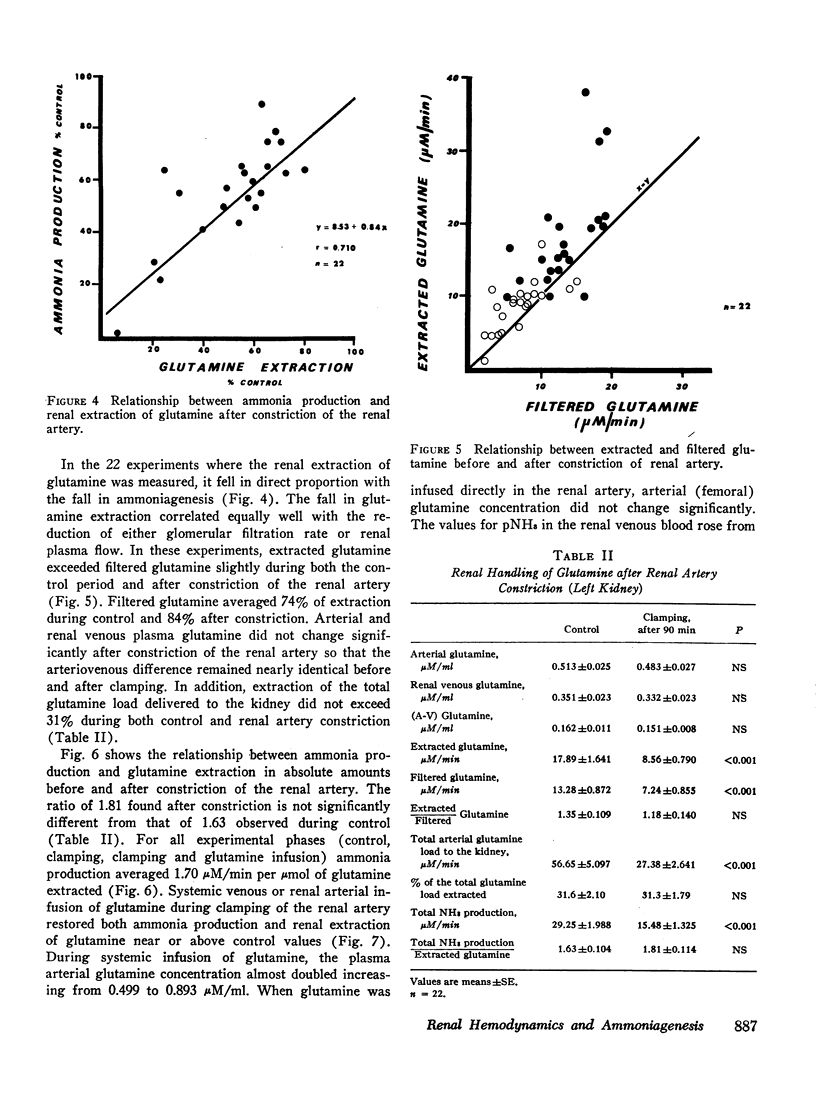
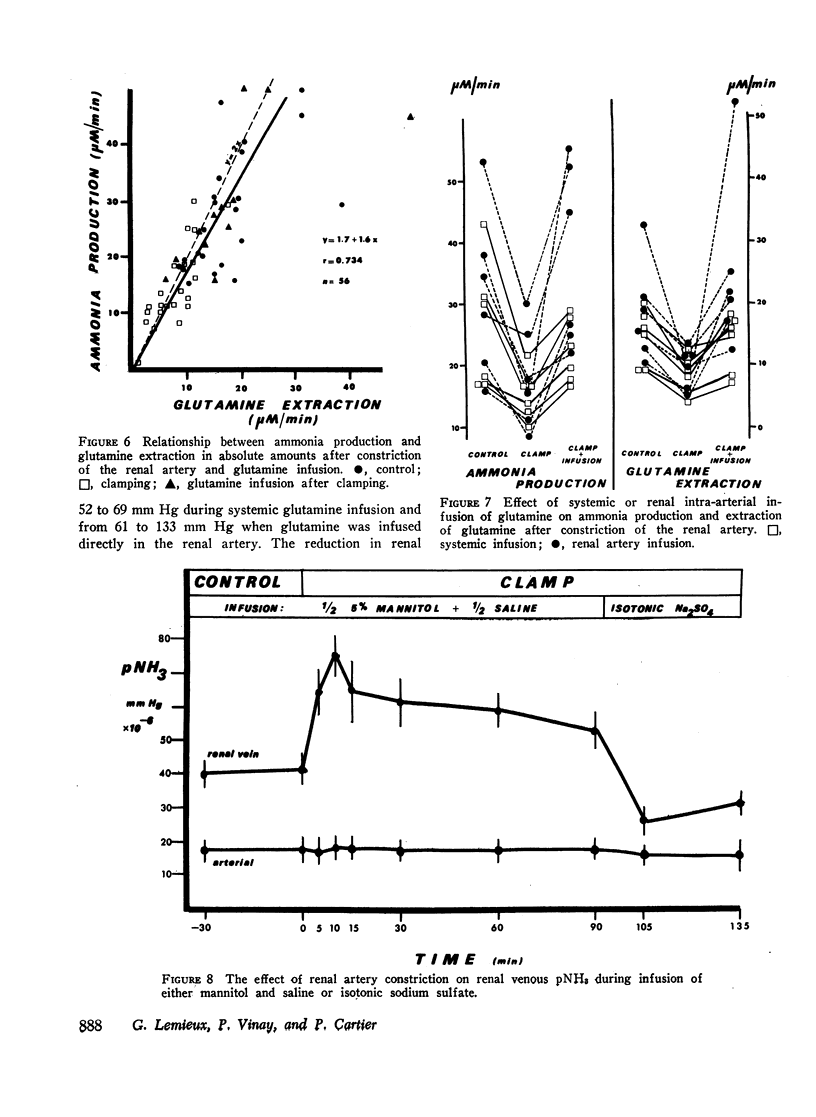
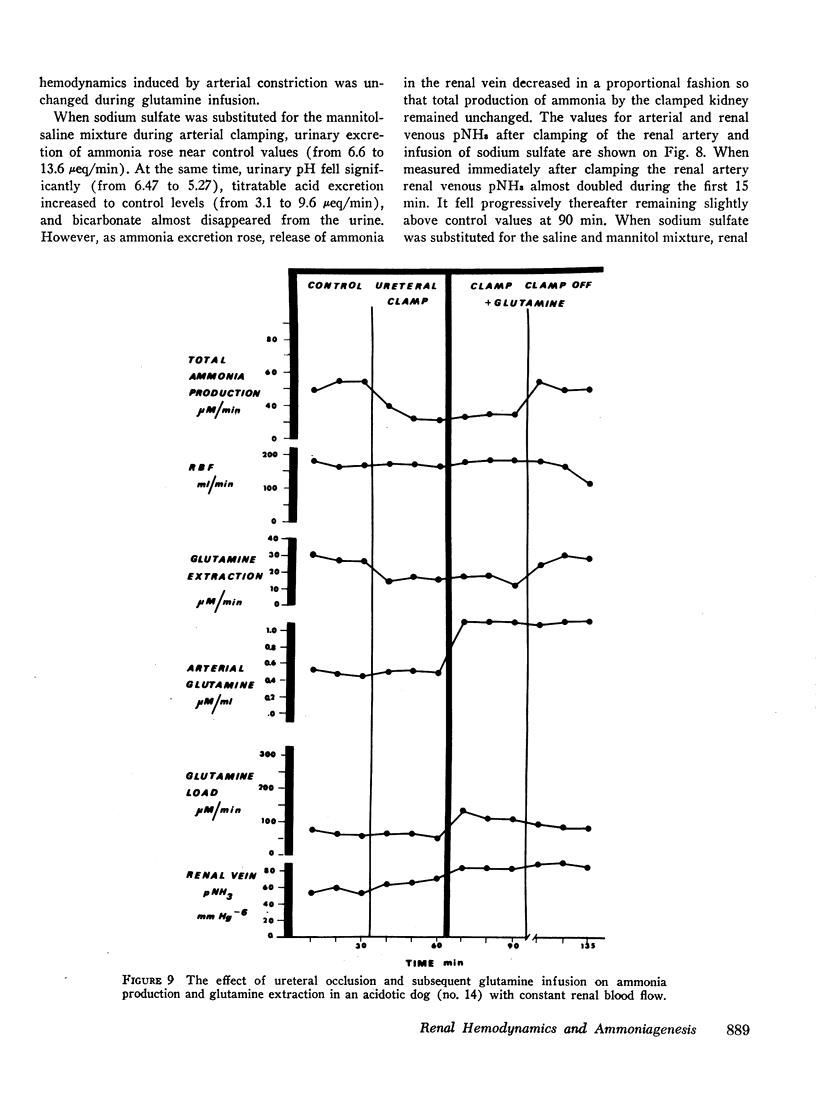
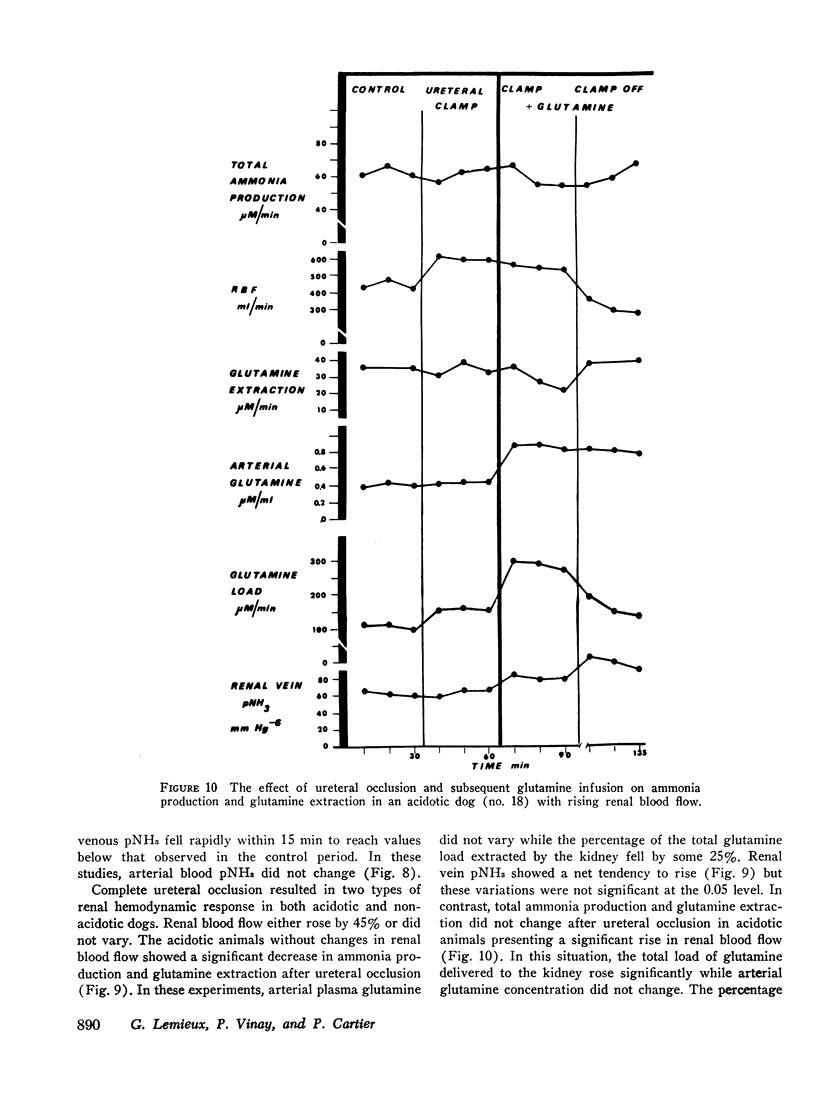
Renal production of ammonia by the left kidney was studied in 31 acidotic dogs (NH4Cl) after acute constriction of the renal artery. Renal ammoniagenesis fell in direct proportion with the reduction in glomerular filtration rate and renal plasma flow. The renal extraction of glutamine by the experimental kidney fell in direct proportion with the reduction in renal hemodynamics. Extracted glutamine remained greater than filtered glutamine indicating that both the luminal and antiluminal transport sites were operative. The relationship between renal extraction of glutamine and ammoniagenesis observed during control was maintained after renal artery constriction (1.7 μmol NH3 produced for each μmol of glutamine extracted). Systemic venous or renal intra-arterial infusion of glutamine during arterial constriction increased renal production of ammonia to or above control values. These observations indicate that the mechanisms responsible for glutamine extraction and ammonia production were operating normally despite reduced hemodynamics. When measured immediately after arterial clamping, the renal venous pNH3 was found to rise significantly decreasing progressively thereafter towards control values. The extracted fraction of total glutamine delivered to the kidney (31%) did not change after acute reduction of the glutamine load. Thus, the antiluminal extraction site was incapable of lowering renal venous plasma glutamine concentration below 0.33 μM/ml. In a second series of experiments, the properties of the antiluminal site of transport for glutamine were studied after complete occlusion of the left ureter in acidotic and nonacidotic animals. Under these circumstances, it was demonstrated that the antiluminal site is capable of extracting sufficient glutamine to maintain total ammonia production at 60% or more of control. In acidotic animals, changes in cellular pNH3 appeared to play a key role on the antiluminal extraction of glutamine since the significant rise in renal blood flow often observed after ureteral occlusion prevented the rise in pNH3 noted when blood flow remained constant. Thus, when renal blood flow rose glutamine extraction and ammonia production were maintained at control values. In these acidotic animals, glutamine infusion failed to influence ammonia production until luminal transport was restored by release of ureteral clamp and resumption of glomerular filtration. The latter observation establishes that reabsorbed glutamine is utilized at least in part for ammonia production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aber G. M., Morris L. O., Housley E., Harris A. M. Bidirectional release of ammonium by the kidneys in patients with respiratory failure. Effect of increasing the concentration of inspired oxygen. Nephron. 1965;2(3):148–158. doi: 10.1159/000179400. [DOI] [PubMed] [Google Scholar]
- DENIS G., PREUSS H., PITTS R. THE PNH3 OF RENAL TUBULAR CELLS. J Clin Invest. 1964 Apr;43:571–582. doi: 10.1172/JCI104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACQUEZ J. A., POPPELL J. W., JELTSCH R. Solubility of ammonia in human plasma. J Appl Physiol. 1959 Mar;14(2):255–258. doi: 10.1152/jappl.1959.14.2.255. [DOI] [PubMed] [Google Scholar]
- Lemieux G., Vinay P., Robitaille P., Plante G. E., Lussier Y., Martin P. The effect of ketone bodies on renal ammoniogenesis. J Clin Invest. 1971 Sep;50(9):1781–1791. doi: 10.1172/JCI106668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. L., Pitts R. F. Species differences in renal glutamine synthesis in vivo. Am J Physiol. 1969 Jan;216(1):117–122. doi: 10.1152/ajplegacy.1969.216.1.117. [DOI] [PubMed] [Google Scholar]
- MALVIN R. L., WILDE W. S., SULLIVAN L. P. Localization of nephron transport by stop flow analysis. Am J Physiol. 1958 Jul;194(1):135–142. doi: 10.1152/ajplegacy.1958.194.1.135. [DOI] [PubMed] [Google Scholar]
- Murphy G. P., Scott W. W. The renal hemodynamic response to acute and chronic ureteral occlusions. J Urol. 1966 May;95(5):636–657. doi: 10.1016/S0022-5347(17)63514-6. [DOI] [PubMed] [Google Scholar]
- Navar L. G., Baer P. G. Renal autoregulatory and glomerular filtration responses to gradated ureteral obstruction. Nephron. 1970 Jul;7(4):301–316. doi: 10.1159/000179831. [DOI] [PubMed] [Google Scholar]
- OMACHI A., MACEY R. I. Intratubular fluid movement in dog kidney during stop flow. Proc Soc Exp Biol Med. 1959 Jun;101(2):386–388. doi: 10.3181/00379727-101-24951. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., JOHNSON J. H., TYOR M. P. The effect of induced hyperammonemia on renal ammonia metabolism. J Clin Invest. 1961 Feb;40:215–221. doi: 10.1172/JCI104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelert H., Nagel W. Die Abhängigkeit der Ammoniakproduktion von der GFR bei Ureterabklemmung und bei Durchblutungsdrosselung in der Hundeniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(2):129–139. [PubMed] [Google Scholar]
- PILKINGTON L. A., WELCH J., PITTS R. F. RELATIONSHIP OF PNH3 OF TUBULAR CELLS TO RENAL PRODUCTION OF AMMONIA. Am J Physiol. 1965 Jun;208:1100–1106. doi: 10.1152/ajplegacy.1965.208.6.1100. [DOI] [PubMed] [Google Scholar]
- PITTS R. F., DEHAAS J., KLEIN J. Relation of renal amino and amide nitrogen extraction to ammonia production. Am J Physiol. 1963 Feb;204:187–191. doi: 10.1152/ajplegacy.1963.204.2.187. [DOI] [PubMed] [Google Scholar]
- PITTS R. F., PILKINGTON L. A., DEHAAS J. C. N15 TRACER STUDIES ON THE ORIGIN OF URINARY AMMONIA IN THE ACIDOTIC DOG, WITH NOTES ON THE ENZYMATIC SYNTHESIS OF LABELED CLUTAMIC ACID AND GLUTAMINES. J Clin Invest. 1965 May;44:731–745. doi: 10.1172/JCI105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington L. A., Young T. K., Pitts R. F. Properties of renal luminal and antiluminal transport of plasma glutamine. Nephron. 1970;7(1):51–60. doi: 10.1159/000179807. [DOI] [PubMed] [Google Scholar]
- Pitts R. F., Pilkington L. A. The relation between plasma concentrations of glutamine and glycine and utilization of their nitrogens as sources of urinary ammonia. J Clin Invest. 1966 Jan;45(1):86–93. doi: 10.1172/JCI105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F., Stone W. J. Renal metabolism of alanine. J Clin Invest. 1967 Apr;46(4):530–538. doi: 10.1172/JCI105554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAYRE F. W., ROBERTS E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958 Nov;233(5):1128–1134. [PubMed] [Google Scholar]
- SCHWARTZ W. B., JENSON R. L., RELMAN A. S. Acidification of the urine and increased ammonium excretion without change in acid-base equilibrium: sodium reabsorption as a stimulus to the acidifying process. J Clin Invest. 1955 May;34(5):673–680. doi: 10.1172/JCI103117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKHAM J. E., SHARMA G. P. ENDOGENOUS AMMONIA FORMATION IN EXPERIMENTAL RENAL ISCHAEMIA. Lancet. 1965 Jan 23;1(7378):195–198. doi: 10.1016/s0140-6736(65)90978-5. [DOI] [PubMed] [Google Scholar]


