Abstract
To better understand the tissue distribution and activity of enzymes involved in angiotensin II (Ang II) processing, we developed a novel molecular imaging method using matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry. Mouse kidney sections (12 μm) were incubated with 10–1,000 μmol/l Ang II for 5–15 min at 37°C. The formed peptides Ang III and Ang-(1–7) were identified by MALDI-TOF/TOF. A third metabolite, Ang-(1–4), was generated from further degradation of Ang-(1–7). Enzymatic processing of Ang II was dose and time dependent and absent in heat-treated kidney sections. Distinct spatial distribution patterns (pseudocolor images) were observed for the peptides. Ang III was localized in renal medulla, whereas Ang-(1–7)/Ang-(1–4) was present in cortex. Regional specific peptide formation was confirmed using microdissected cortical and medullary biopsies. In vitro studies with recombinant enzymes confirmed activity of peptidases known to generate Ang III or Ang-(1–7) from Ang II: aminopeptidase A (APA), Ang-converting enzyme 2 (ACE2), prolyl carboxypeptidase (PCP), and prolyl endopeptidase (PEP). Renal medullary Ang III generation was blocked by APA inhibitor glutamate phosphonate. The ACE2 inhibitor MLN-4760 and PCP/PEP inhibitor Z-pro-prolinal reduced cortical Ang-(1–7) formation. Our results establish the power of MALDI imaging as a highly specific and information-rich analytical technique that will further aid our understanding of the role and site of Ang II processing in cardiovascular and renal pathologies.
Keywords: matrix-assisted laser desorption ionization imaging, angiotensin-converting enzyme 2, prolyl carboxypeptidase, prolyl endopeptidase, aminopeptidase A
the potent vasoconstrictor angiotensin ii (Ang II) is the main effector peptide of the renin-angiotensin system (RAS), controlling renal function and blood pressure. Inhibitors of Ang II synthesis and receptor binding are commonly used in the management of hypertension, congestive heart failure, and renal disease. However, there is growing evidence that therapeutic interventions targeting receptor binding and peptide generation have not provided as much cardiovascular risk reduction as anticipated (1, 56). The search for treatment alternatives continues, particularly since reports have emerged of novel Ang II binding sites as well as reactivation of Ang II synthesis after chronic treatment with Ang-converting enzyme (ACE) inhibitors (4, 32, 41, 44). Ang II degradation might be a target for potential new treatment strategies against Ang II's vasoconstrictive actions (16, 42). However, this requires a reliable method to assess Ang II processing enzyme activities, particularly in pathological states shown to be associated with up/downregulation of RAS enzymes (7, 15, 59, 61). Recognizing the advantages of mass spectrometric (MS) detection for enzyme assays (25), our laboratory developed surface-enhanced laser desorption ionization-time-of-flight MS assays for ACE activities (ACE and ACE2) using natural peptide substrates (17, 18). These studies used kidney and brain extracts and neat plasma. Results for ACE2 activity documented absence of plasma ACE2 in mice under basal conditions and lack of kidney ACE2 activity in ACE2-knockout mice. This novel MS approach showed high specificity and allowed for determination of possible additional proteolytic cleavage sites while the natural peptide substrates were used.
The next step was the development of an in situ tissue imaging approach for the RAS using matrix-assisted laser desorption ionization (MALDI)-MS coupled with tissue incubation with natural peptide precursors. The method combines the advantages of MS detection with anatomic specificity for measurement of enzyme activities. MALDI imaging was established initially for the visualization and in situ identification of molecules present on or near the tissue surface (8, 10, 48, 49). To date, MALDI imaging and profiling of peptides and proteins have been applied to a wide variety of tissues (37). We explored this novel imaging method with the goal of characterizing and localizing RAS enzyme activities involved in renal Ang II processing. Described herein is the power of a new, dynamic MS technique as a highly specific and information-rich analytical approach that is not restricted to the renal RAS but can be used to address a variety of questions related to enzymatic function in biological tissues.
METHODS
Animals and tissue samples.
Male C57BL/6 mice were purchased from a commercial source (Harlan, Indianapolis, IN). Animals were housed at 22°C under a 12:12-h light-dark cycle with ad libitum access to water and standard mouse chow (3.0 kcal/g, 40.6% carbohydrate, 5.5% fat, and 22% protein; Harlan-Teklad, Madison, WI). Mice (12–16 wk old) were decapitated, and kidneys were quickly removed and frozen in liquid nitrogen. This procedure from decapitation to freezing of kidneys was accomplished in <30 s. Kidneys were stored at −80°C until they were used for testing. All experimental protocols were approved by the Wright State University Animal Care and Use Committee.
Tissue sectioning.
Kidney halves (sagittal) were placed on the cryostat chuck and embedded with a small amount of freezing medium (Triangle Biomedical Sciences, Durham, NC). Sections (12 μm) were cut on a cryostat at −20°C. Kidney sections were thaw-mounted onto prechilled indium-tin-oxide coated glass slides and then desiccated at room temperature for 15–20 min followed by incubation with Ang II.
In situ enzyme activity.
Kidney sections were incubated with 10–1,000 μmol/l Ang II (dissolved in water) at 37°C for 5–15 min. In control experiments, sections were autoclaved for 15 min at 121°C to destroy enzyme activities. For each time point and substrate concentration, two sections were analyzed. In Ang III and Ang-(1–7) degradation studies, kidney sections were incubated with 100 μmol/l peptide solution (37°C) for 1–5 min. Inhibition of renal Ang III and Ang-(1–7) formation was tested using reaction mixtures that contained 100 or 1,000 μmol/l Ang II and inhibitor: glutamate phosphonate [aminopeptidase A (APA) inhibitor, a gift from Dr. Robert Speth of Nova Southeastern University, Fort Lauderdale, FL], MLN-4760 (ACE2 inhibitor, a gift from the former Millennium Pharmaceuticals, Cambridge, MA), and ZPP [prolyl carboxypeptidase (PCP)/prolyl endopeptidase (PEP) inhibitor; Enzo Life Sciences International, Plymouth Meeting, PA]. After incubation with peptide solutions, tissue sections were desiccated at room temperature for 20 min.
MALDI matrix application.
MALDI matrix consisting of 10 mg/ml α-cyano-4-hydroxy-cinnamic acid in 60% HPLC-grade methanol, 10% HPLC-grade acetone, and 0.3% sequencing-grade trifluoroacetic acid was spray-coated onto kidney tissue sections using a thin-layer chromatography nebulizer with nitrogen gas (10 psi). The α-cyano-4-hydroxy-cinnamic acid solution was repeatedly sprayed across the sections from a distance of 20 cm, allowing for 30 s of drying time between three passes until a uniform matrix coating was achieved. Degree of matrix coating was analyzed using light microscopy. Spray-coated tissue sections were desiccated at room temperature for 5–10 min prior to MALDI analysis.
MALDI imaging.
MALDI images were obtained using an Autoflex III smartbeam MALDI time-of-flight (TOF)/TOF instrument (Bruker Daltonics). The mass spectrometer was operated with positive polarity in reflectron mode. A total of 200 spectra were acquired for each spot in the range of mass-to-charge ratio (m/z) of 500–3,000 at a laser frequency of 100 Hz. A 200-μm raster was used, which was aligned to an optical image. The spectral analysis was performed with proprietary Bruker Flex Analysis and Imaging software. Spectra were mass calibrated by collecting 200 laser shots of spots containing Bruker peptide calibration standard II, consisting of nine peptide standards covering a mass range of 700–3,200 Da. Mass spectra were baseline subtracted (Top Hat), smoothed (2 m/z width, 4 cycles; Gauss), and normalized using the parameters of the Bruker FlexImaging software (Ymean/Ymax Threshold 0.5). Unknown ion peaks were fragmented using the Bruker LIFT method (50) and identified upon comparison with standard peptides.
In summary, essential steps that must be controlled during sample preparation to obtain optimal results using the imaging approach are 1) quick removal and rapid freezing of organs after collection, 2) avoidance of localized tissue warming and contamination of tissue-cutting surface with freezing medium, 3) care in applying the peptide solution such that the tissue is not damaged, 4) minimal movement of slides during incubation and transfer to desiccator, and 5) uniform matrix coating.
Ang II peptide processing in renal medulla and cortex punch biopsies.
Frozen kidneys were sliced coronally (1-mm sections) using an acrylic slicer matrix. Biopsies from medulla and cortex were collected using a prechilled 1.5-mm stainless-steel punch (Integra Miltex, York, PA). Three medulla and three cortex tissue pieces from one kidney were combined and homogenized as 10% suspensions in Lysis-M EDTA-Free buffer (Roche Applied Science) using a precellys 24-tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). The homogenate was centrifuged at 10,000 g for 5 min to remove cellular debris. Total protein content was determined in the supernatant using the Bradford protein assay with BSA as a standard (Bio-Rad Protein Assay Reagent). Protein samples (100 μg) were incubated with 1 mmol/l Ang II for 15 min at 37°C. In control assays, 1 mmol/l Ang II was incubated without protein in Lysis-M EDTA-Free buffer for 15 min at 37°C. The reaction was acidified by adding trifluoroacetic acid (TFA; final concentration 1%) and dried for 4 h using a vacuum concentrator. The extracts were reconstituted in 0.1% TFA and loaded onto a SepPak C18 cartridge (Waters) that had been preconditioned with two 1-ml portions of 80% methanol containing 0.1% TFA and two 1-ml portions of 0.1% TFA. The cartridge was washed twice with 1 ml of 0.1% TFA and 1 ml of water. Peptides were eluted with 80% methanol containing 0.1% TFA, dried with a vacuum concentrator, and resuspended in 90% acetonitrile containing 0.3% TFA. Samples were directly injected (flow rate of 200 μl/h) into an HCT Ultra ion trap with an electrospray ion source (Bruker Daltonics). For detection of Ang peptides, the ion trap was operated with positive polarity in standard enhanced scan mode from m/z 50 to 3,000. The ion source parameters were set as follows: nebulizer pressure, 10 psi; dry temperature, 300°C; and dry gas flow, 5 l/min.
In vitro enzyme activity.
Recombinant APA, ACE2, PCP, and PEP were purchased from R & D Systems (Minneapolis, MN). Enzymes (50 ng) were incubated for 1 h at 37°C with 50 μM Ang II in buffer prepared according to the manufacturer's protocol. The reaction was stopped by acidification with TFA (final concentration 1%) and dried using a vacuum concentrator. The assays were reconstituted in 0.1% TFA, and peptides were loaded onto a μ-C18 Ziptip (Millipore) that had been activated with 90% acetonitrile and 0.1% TFA. The Ziptip was washed with 0.1% TFA and the sample eluted in 90% acetonitrile containing 0.3% TFA. For each enzyme, a total of 10 assays were purified and combined. Samples were analyzed using an HCT Ultra ion trap, as described in Ang II peptide processing in renal medulla and cortex punch biopsies.
Data analysis.
MALDI images were quantitatively evaluated using MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA). Signals represented in color on pseudocolor images were quantitated as integrated intensity and normalized to the area of the whole kidney section. All data are expressed as means ± SE.
RESULTS
Method optimization of MALDI imaging for Ang peptides in kidney.
MALDI has been widely used for the detection of peptides and proteins in tissues and biological fluids (3, 8, 28, 34). In our studies, the MALDI laser is set to automatically screen tissue sections, producing an ordered array of mass spectra with information on nominal m/z values (8). Pseudocolor images are then created by integrating the m/z peak areas and plotting the relative values using a color scale (48). Our goal was to use MALDI imaging for characterization and localization of Ang II processing in kidney sections. The first step was to establish and validate the method for matrix application and to determine the peptide dose response in tissue sections. To evaluate the dependency of the MS signal intensity on matrix coating, three mouse kidney sections were spotted randomly with 500 ng of Ang III or Ang-(1–7), the two main Ang II metabolites, and spray-coated with increasing amounts of MALDI matrix. Peptide intensity was calculated for each peptide spot and normalized to the entire area of the kidney section. Peptide signals were optimal with 12 or more spraying cycles (Fig. 1, A and B), and light microscopy confirmed a continuous, even layer of matrix (Fig. 1C). To determine the linearity of MS signal intensities, 25–250 ng of Ang III and Ang-(1–7) were spotted randomly onto kidney sections and analyzed by MALDI imaging. Quantitation of signal intensities revealed that MS signals for the peptide standards Ang III and Ang-(1–7) were reproducible and linearly dependent on peptide concentration in a directly proportional manner (Fig. 1, D and E). The assay limit of detection was ∼5 ng for all Ang peptides. This is similar to the approach of Nilsson et al. (39) for the drug standard tiotropium.
Fig. 1.
Optimization of matrix-assisted laser desorption ionization (MALDI) imaging for angiotensin (Ang) III and Ang-(1–7) in kidney sections. Sections (12 μm) were obtained from fresh frozen mouse kidneys. Peptide standards were spotted onto kidney sections. A: dependency of Ang III signal intensities on matrix coating. B: dependency of Ang-(1–7) signal intensities on matrix coating. C: light microscopic image of matrix coating. D: MALDI dose-response curve for Ang III. E: MALDI dose-response curve for Ang-(1–7).
In situ MALDI imaging of renal Ang II metabolism.
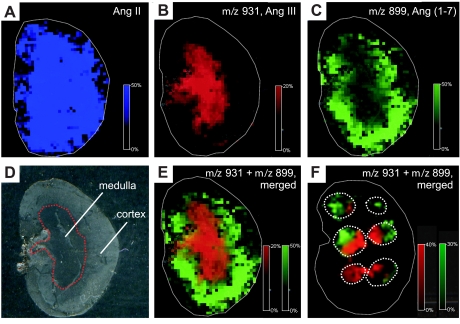
Ang II metabolism in kidney sections was documented by measurement of peptide formation, amount, and tissue localization. Mouse kidney sections were incubated with 10 μl of 1,000 μmol/l Ang II for 15 min, followed by analysis using MALDI imaging. The resulting array of mass spectra was screened for newly formed peptides generated on the kidney section from Ang II. Two new peaks with m/z 931 and m/z 899 were detected, consistent with the molecular masses of Ang III and Ang-(1–7), respectively (Fig. 2). These peptides were absent in kidney sections not incubated with Ang II. In addition, the reaction was destroyed by heat treatment, confirming that the processing was of enzymatic origin. The product m/z 931 was localized predominantly in the renal medulla, whereas the second peptide with m/z 899 was detected mainly in renal cortex. An overlay of the MS signals obtained for the two peptide products illustrates their distinct spatial distribution patterns (Fig. 2E). This clear differentiation of Ang III and Ang-(1–7) formation was also detected in 15-min incubations with Ang II peptide spots (1,000 μmol/l) placed randomly across the kidney section (Fig. 2F). A search for other Ang peptide species (Table 1), as expected from further degradation, revealed detection of m/z 552 in renal cortex. This peptide was identified as Ang-(1–4), a biologically inactive tetrapeptide generated from Ang-(1–7) by neprilysin (2).
Fig. 2.
MALDI imaging of Ang II and metabolites generated on kidney sections. A: mass spectrometric (MS) signal for Ang II [mass-to-charge (m/z) 1,046] before incubation. B: MS signal for m/z 931 detected after 15 min of incubation with 1 mmol/l Ang II. C: MS signal for m/z 899 detected after 15 min of incubation with 1 mmol/l Ang II. D: photographic image of a kidney section. E: overlay of both MS signals m/z 931 and m/z 899. F: kidney section spotted 6 times with 1 mmol/l Ang II and incubated for 15 min. Overlay of both MS signals m/z 931 and m/z 899.
Table 1.
Theoretical and observed ion mass for Ang II metabolic assay
| Ang Species | Amino Acid Sequence | Theoretical Mass (m/z) | Observed Mass (m/z) |
|---|---|---|---|
| Ang II | DRVYIHPF | 1,046.2 | 1,046.2 |
| Ang III | RVYIHPF | 931.1 | 931.0 |
| Ang-(1–7) | DRVYIHP | 899.0 | 899.0 |
| Ang IV | VYIHPF | 774.9 | <LOD |
| Ang-(1–5) | DRVYI | 664.8 | <LOD |
| Ang-(1–4) | DRVY | 551.6 | 551.6 |
| Ang-(5–8) | IHPF | 512.6 | <LOD |
Ang, angiotensin; m/z, mass-to-charge ratio; LOD, limit of detection (∼5 ng).
Identification of metabolites m/z 931, m/z 899, and m/z 552.
Figure 2 shows the localization pattern for peptides generated from Ang II but not their precise structure. The next important step was identification and quantification of the Ang II metabolites formed in mouse kidney. The chemical nature of the generated peptides was verified by MS/MS, in which the precursor ion of interest is selected and fragmented into ions that are subsequently analyzed. Using the MALDI LIFT-TOF/TOF function of the Bruker Autoflex III MS instrument (50), we first obtained MS/MS spectra for synthetic peptides Ang III, Ang-(1–7), and Ang-(1–4) spotted onto kidney sections (Fig. 3). These MS/MS spectra were identical to the MS/MS spectra of m/z 931, m/z 899, and m/z 552 generated enzymatically in mouse renal medulla and cortex (Fig. 3).
Fig. 3.
Identification of Ang III and Ang-(1–7) by MALDI-time of flight (TOF)/TOF as 2 metabolites generated from Ang II in mouse kidney. A: MS/MS of Ang III standard. B: MS/MS of the enzymatic product m/z 931. C: MS/MS of Ang-(1–7) standard. D: MS/MS of the enzymatic product m/z 899. E: MS/MS of Ang-(1–4) standard. F: MS/MS of the enzymatic product m/z 552.
Reproducibility of renal Ang II metabolic assays.
To apply the metabolic assays to pathological testing, it was important to quantify peptide levels and establish reproducibility. Peptide formation on consecutive kidney sections obtained from a single animal (intra-animal variation) and kidney sections obtained from different animals (interanimal variation) was evaluated for reproducibility of the newly established MALDI imaging technique. There was little variation for Ang III and Ang-(1–7) forming enzymatic activities within (19.4 ± 1.9 and 13.1 ± 2.0 intensity/area, respectively) or between animals (20.9 ± 1.6 and 12.7 ± 2.0 intensity/area, respectively).
Effect of time and substrate concentration on peptide formation.
Distinct Ang III and Ang-(1–7) product profiles were observed in kidney sections incubated with various Ang II concentrations, 10–1,000 μmol/l, for 5 and 15 min (Fig. 4). At low Ang II concentrations, formation of Ang III increased with incubation time, whereas Ang-(1–7) decreased likely because of a high efficiency of enzymes degrading the formed peptide. At high Ang II concentrations, both Ang III and Ang-(1–7) formation increased with incubation time. In this set of experiments, there was again consistency in the localization pattern: Ang III in the medulla and Ang-(1–7) in the cortex.
Fig. 4.
Dependency of Ang III and Ang-(1–7) formation on incubation time and Ang II substrate concentrations. Kidney sections were incubated with varying concentrations of Ang II (10–1,000 μmol/l) for 5 and 15 min. Metabolism of Ang II and formation of Ang III/Ang-(1–7) peptides were analyzed by MALDI imaging. A: presence of Ang II after incubation of kidney sections with 10–1,000 μmol/l Ang II for 5 and 15 min. B: formation of Ang III after 5 and 15 min of incubation with 10–1,000 μmol/l Ang II. C: formation of Ang-(1–7) after 5 and 15 min of incubation with 10–1,000 μmol/l Ang II. Note that bars are absent for 10, 50, and 100 μmol/l in A and C.
Ang III and Ang-(1–7) degradation in kidney.
These distinct spatial distribution patterns were further analyzed by a study of peptide degradation. Kidney sections were incubated with 100 μmol/l Ang III or 100 μmol/l Ang-(1–7) for 1–5 min. Ang III was rapidly degraded in renal cortex but was still detectable in medulla after 5 min. Ang-(1–7) was degraded much more slowly, and there was no difference between cortex and medulla (Fig. 5).
Fig. 5.
Degradation of Ang III and Ang-(1–7) in mouse kidney sections.
Complementary studies of Ang II peptide processing.
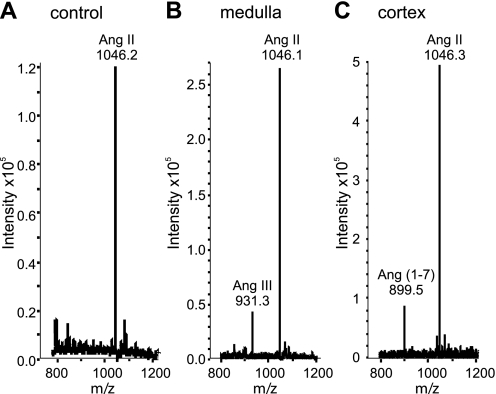
The quality of MALDI-MS signals depends highly on matrix composition and sample preparation (11). MALDI imaging carries the potential risk of ionization biases within a heterogenous tissue (31). To further validate the MALDI imaging results, we examined Ang II processing in renal cortex and medulla, using punch biopsies taken from kidney sections coupled with peptide purification and a complementary mass spectrometric approach. In 100-μl test assays, renal medulla and cortex biopsy homogenates (100 μg) were incubated with 1 mmol/l Ang II for 15 min. The incubation mixtures were purified by C18 solid-phase extraction and injected directly into an HCT Ultra ion trap MS. A signal for m/z 931 but not m/z 899 was detected in the medulla enzyme assays, whereas cortex enzyme assays showed a peak only with m/z 899, confirming the MALDI imaging results (Fig. 6).
Fig. 6.
Complementary studies of Ang II peptide processing. A: MS signal for 1,000 μmol/l Ang II incubated for 15 min at 37°C in lysis buffer without kidney protein. B: MS of 1,000 μmol/l Ang II incubated for 15 min at 37°C with medulla protein (100 μg) obtained from kidney punch biopsies. C: MS of 1,000 μmol/l Ang II incubated for 15 min at 37°C with cortex protein (100 μg) obtained from kidney punch biopsies.
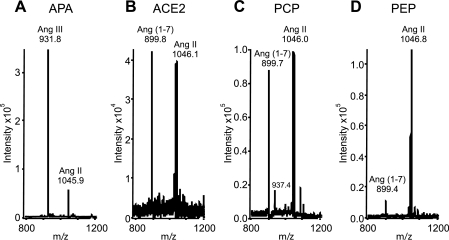
Identification of Ang III and Ang-(1–7) forming enzymes.
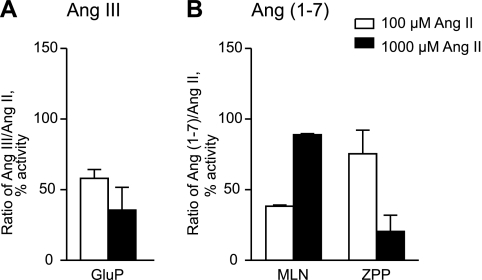
APA (EC 3.4.11.7) has been shown to form Ang III through hydrolysis of the Asp1-Arg2 peptide bond of Ang II (33). Three different enzymes, ACE2 (EC 3.4.17.23), PEP (EC 3.4.21.26), and PCP (EC 3.4.16.2), are known to generate Ang-(1–7) from Ang II through COOH-terminal cleavage of the Pro7-Phe8 bond of Ang II (30, 51, 53, 55, 57). We first confirmed activity of recombinant APA, ACE2, PCP, and PEP with Ang II as substrate (Fig. 7) and further characterized Ang II processing in kidney using peptidase inhibitors. Amastatin, a general inhibitor of APA (52), did not prevent Ang III formation under any conditions. Therefore, kidney sections were incubated with 100 or 1,000 μmol/l Ang II for 5 or 15 min, respectively, in the presence of the specific APA inhibitor glutamate phosphonate (GluP) (35). Analysis by MALDI imaging revealed that GluP (3 μmol/l) effectively inhibited Ang III formation ≤60% (Fig. 8A).
Fig. 7.
Ang II conversion in incubations with recombinant protein (50 ng). A: MS of Ang III formation by aminopeptidase A (APA). B: MS of Ang-(1–7) formation by Ang-converting enzyme 2 (ACE2). C: MS of Ang-(1–7) formation by prolyl carboxypeptidase (PCP). D: MS of Ang-(1–7) formation by prolyl endopeptidase (PEP).
Fig. 8.
Inhibitor effects on renal Ang III and Ang-(1–7) formation. Inhibitors were added to 100 or 1,000 μmol/l Ang II, and kidney sections were incubated with reaction mixtures for 5 or 15 min, respectively. A: effect of 3 μmol/l glutamate phosphonate (GluP; APA inhibitor) on renal Ang III formation. B: effect of 1 μmol/l MLN-4760 (MLN; ACE2 inhibitor) or 10 μmol/l Z-pro-prolinal (ZPP; PCP/PEP inhibitor) on Ang-(1–7) formation. Ang III or Ang-(1–7) formation without addition of inhibitors was set at 100%.
Next, two inhibitors were chosen to test for their effects on renal Ang-(1–7) formation: MLN-4760 (MLN), a specific ACE2 inhibitor (14), and Z-polyl-prolinal (ZPP), a PCP and PEP inhibitor (51, 58). At 100 μmol/l Ang II and 5 min of incubation time, Ang-(1–7) formation was inhibited by MLN (1 μmol/l) to 38 ± 1% but not by ZPP (10 μmol/l) (Fig. 8B). A minimal effect on enzyme activity by the PCP/PEP inhibitor suggests ACE2-independent Ang-(1–7) formation at low Ang II concentrations. However, PCP/PEP involvement in Ang-(1–7) formation was more evident with higher Ang II (1,000 μmol/l) concentrations and at 15 min of incubation time. This is documented in Fig. 8B, which shows that the response to the specific inhibitors MLN and ZPP was switched in the face of higher Ang II levels (1,000 μmol/l). MLN (1 μmol/l) had no effect on enzyme activity, whereas ZPP (10 μmol/l) produced an inhibition of 80 ± 12% (Fig. 8B). In conclusion, assays that used <100 μmol/l Ang II assessed ACE2 activity, whereas assays that used 1,000 μmol/l Ang II detected PCP/PEP activity.
DISCUSSION
This study describes the development of a reliable and highly specific method that uses MALDI imaging for the determination of Ang II metabolites and Ang II processing activity in regions of mouse kidney. MALDI imaging of kidney sections incubated with Ang II detected three peptides with m/z 931, m/z 899, and m/z 552. The two main Ang II products were identified as Ang III and Ang-(1–7) using MALDI-TOF/TOF. The peptide with m/z 552 was assigned to Ang-(1–4), a biologically inactive degradation product of Ang-(1–7). Ang III was localized predominantly in renal medulla, whereas Ang-(1–7) and Ang-(1–4) were detected mainly in cortex. This clear differentiation of Ang II peptide processing was confirmed in incubations with Ang II spotted across the kidney section as well as in a complementary MS approach. Validation of the MALDI imaging technique showed a linear signal dose response for Ang peptides, and only minimal variations were observed within and between animals, indicating good reproducibility. Formation of Ang III and Ang-(1–7) was absent in heat-treated kidney sections and dependent on incubation time and Ang II concentrations. Under conditions of high substrate concentration and at longer incubation times, elevated amounts of Ang II metabolites were detected. At short time periods and high Ang II concentrations, lower Ang-(1–7) and Ang III levels were found, which was likely due to a high efficiency of enzymes degrading the formed peptides. Studies of peptide degradation showed that Ang III was more rapidly degraded in renal cortex compared with medulla, whereas Ang-(1–7) was degraded at similar rates in cortex and medulla. The enzyme reactions leading to Ang III and Ang-(1–7) formation were further characterized. We first confirmed activity of recombinant APA, ACE2, PCP, and PEP with Ang II. Use of specific inhibitors GluP (APA inhibitor), MLN (ACE2 inhibitor), and ZPP (PCP/PEP inhibitor) indicated that APA is responsible for medullary Ang III formation, whereas ACE2 and PCP/PEP generate cortical Ang-(1–7).
Ang III and Ang-(1–7) are involved in the regulation of blood pressure and renal function (20). The observed spatial distribution patterns of the Ang II metabolites suggest regionally localized biological actions. Ang III possesses effects and receptor-binding affinities similar to those observed for Ang II, suggesting that this heptapeptide may be equally or even more important than Ang II in some actions, e.g., aldosterone or vasopressin release (60, 63). Indeed, there is a drug development that targets Ang III formation with the idea that it might be a treatment strategy for certain forms of hypertension (5, 6). Our results document an abundance of Ang III formation in the medulla, providing support for a pathway by which Ang III might interact to affect aldosterone and vasopressin.
Ang-(1–7) is thought to oppose the deleterious actions of Ang II, causing vasodilation and antiproliferation and perhaps mediating other beneficial cardiovascular and renal actions (20). Indeed, it has been postulated that ACE2 is renoprotective due to its highly specific action to generate Ang-(1–7) from Ang II (21, 38, 59, 61, 62). In the kidney, the highest levels of ACE2 and the proposed Ang-(1–7) binding site are found in cortical proximal tubules (13, 27, 40, 62). This is consistent with our results suggesting that ACE2-dependent Ang-(1–7) formation occurs mainly in renal cortex.
Using MALDI imaging, there was no evidence for endogenous Ang peptides in kidney. This is likely due to a low abundance along with the effects of fast-acting degradative enzymes. Endogenous Ang levels reported in literature are <500 fmol/g kidney tissue (9, 22), which is below our MALDI-based limit of detection. In addition, matrix and biological background interferences are known to impair ionization of low-abundance molecules. Mass spectrometry coupled with electrospray ionization and liquid chromatography was the only method to achieve sensitivities needed to detect minute amounts of endogenous Ang peptides in biological tissue (12, 36). The benefit of the MALDI imaging approach is that it does not require pooling of samples and still allows for the localization of enzyme activity within biological specimens. The advantage of the activity method is that a small concentration of active enzyme (ng amounts) is sufficient to convert a substrate into product at levels detectable by MALDI imaging.
Besides limitations in detection sensitivity and sampling efficiency, the imaging approach carries other potential risks related to sample preparation, postmortem degradation of peptides and proteins, ionization bias, and lateral diffusion of analytes during incubation (19, 24, 31, 45, 54). Using multiple conditions and treatment, these issues have been addressed and managed in our study. For matrix application, we developed a simple yet reliable method as described in methods. An automated system is not an absolute requirement. Moreover, compliance to a standardized sample workup protocol and strict observation of postmortem processing times minimized differences and irregularities of MS signals. Although we cannot exclude some postmortem degradation that could change endogenous active enzyme forms, this does not appear to be an important issue given the reproducibility of the results. The problem of ionization biases within heterogenous tissue such as the kidney can be excluded in our study since signal intensities for all Ang peptides were equal over the entire kidney section (Figs. 2A and 5). Scale bars in these figures are set at 50% to obtain brighter color images. Analyzed peptide intensities were within the linear range, thus allowing for a semiquantitative approach. Furthermore, ionization biases can be ruled out since regional-specific peptide formation was confirmed using microdissected cortical and medullary biopsies. Careful attention to detail and consistency throughout the experimental process avoided analyte migration during incubation. This is documented by the clear differentiation of medullary and cortical MS signals. Indeed, previous reports confirm that washes in organic or aqueous solutions did not cause migration of peptides across tissue sections (23, 43).
Ang III-generating enzyme activity was easily detected by MALDI imaging in the inner medullary region of the kidney. Specificity was verified via use of GluP, a specific APA inhibitor. These results are in contrast to earlier reports that showed higher levels of APA in renal cortex than in medulla (29, 46, 47). The regional differences in APA activity may be related to the analytical approaches used for the detection of enzyme activity. Whereas our studies used the natural peptide as a precursor, previous studies tested β-napthylamide derivatives as substrates with spectrophotometric detection. Additionally, soluble and membrane-bound forms of APA were found to be differentially active (15). The MALDI imaging approach visualized active APA, whereas immunological approaches are targeted toward the detection of overall protein located within a tissue.
It was shown previously that PCP and PEP are capable of converting Ang II to Ang-(1–7), albeit with lower catalytic efficiencies than ACE2 (30, 51, 55, 57). Our study with ACE2, PCP, and PEP inhibitors indicates that cortical Ang-(1–7) formation in the presence of low Ang II concentrations is due primarily to the actions of ACE2. However, with higher Ang II concentrations, non-ACE2-dependent mechanisms may contribute to Ang-(1–7) generation. Thus, one must entertain the idea of PCP/PEP-mediated formation of Ang-(1–7), which is dependent on the local cellular milieu. This finding might be of particular interest for studies using chronic infusion of Ang II (1,000 ng·kg−1·min−1 for 4 wk), an established model of hypertension (26).
In conclusion, this study describes a new method for molecular imaging of the renal RAS using MALDI mass spectrometry. It allows for the simultaneous localization of Ang II metabolites and corresponding enzyme activities in kidney regions. Our approach allows for the use of natural substrates and characterization of multiple products. Herein we document the benefit of a MALDI-MS technique that combines specificity and selectivity for multiple analytes of interest with their spatial distribution on tissue sections and correlation to enzyme activity. Further studies applying this technique will elucidate the relevance for the role and site of Ang II processing in establishing RAS balance and cardiovascular and renal health. There are also plans to expand this tissue-imaging technique to Ang I, the physiologically most relevant precursor of Ang II, other tissues, and enzyme systems, as well as drug testing and development.
GRANTS
This work was supported by National Institutes of Health Grant R01-HL-093567 and fellowship 1-F32-DK-093226-01.
DISCLOSURES
No competing interests, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G., K.M.E., D.R.C., and M.M. did the conception and design of the research; N.G. performed the experiments; N.G., K.M.E., D.R.C., and M.M. analyzed the data; N.G., K.M.E., D.R.C., and M.M. interpreted the results of the experiments; N.G. prepared the figures; N.G. and M.M. drafted the manuscript; N.G., K.M.E., D.R.C., and M.M. edited and revised the manuscript; N.G., K.M.E., D.R.C., and M.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Richard Caprioli and Erin Seeley, Vanderbilt University, Nashville, TN, for training and analytical support in developing the MALDI imaging method. We acknowledge William C. Grunwald, Jr., Mary Key, and Naima S. Rodwan for excellent technical assistance. We give special thanks to our colleague Nathan M. Weir for invaluable support. We thank Dr. Robert C. Speth, Nova Southeastern University, Fort Lauderdale, FL, for providing glutamate phosphonate and Dr. Randall A. Skidgel, University of Illinois, Chicago, IL, for thoughtful discussion.
REFERENCES
- 1. Alfie J, Aparicio LS, Waisman GD. Current strategies to achieve further cardiac and renal protection through enhanced renin-angiotensin-aldosterone system inhibition. Rev Recent Clin Trials 6: 134–146, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Arthur JM, Thongboonkerd V, Scherzer JA, Cai J, Pierce WM, Klein JB. Differential expression of proteins in renal cortex and medulla: a proteomic approach. Kidney Int 62: 1314–1321, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opin Pharmacother 8: 529–535, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bodineau L, Frugière A, Marc Y, Claperon C, Llorens-Cortes C. Aminopeptidase A inhibitors as centrally acting antihypertensive agents. Heart Fail Rev 13: 311–319, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bodineau L, Frugière A, Marc Y, Inguimbert N, Fassot C, Balavoine F, Roques B, Llorens-Cortes C. Orally active aminopeptidase A inhibitors reduce blood pressure: a new strategy for treating hypertension. Hypertension 51: 1318–1325, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of Ang-(1–7) and ACE2 during pregnancy. Hypertension 42: 749–753, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem 69: 4751–4760, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Cervenka L, Mitchell KD, Oliverio MI, Coffman TM, Navar LG. Renal function in the AT1A receptor knockout mouse during normal and volume-expanded conditions. Kidney Int 56: 1855–1862, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Chaurand P, Stoeckli M, Caprioli RM. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal Chem 71: 5263–5270, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Cohen SL, Chait BT. Influence of matrix solution conditions on the MALDI-MS analysis of peptides and proteins. Anal Chem 68: 31–37, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Cui L, Nithipatikom K, Campbell WB. Simultaneous analysis of angiotensin peptides by LC-MS and LC-MS/MS: metabolism by bovine adrenal endothelial cells. Anal Biochem 369: 27–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA, Simões e Silva AC, Ribeiro Vieira MA. ACE2-angiotensin-(1–7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 119: 385–394, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, Gavin JM, Hales P, Kaushik VK, Stewart M, Tummino PJ, Vickers CS, Ocain TD, Patane MA. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc 124: 11852–11853, 2002 [DOI] [PubMed] [Google Scholar]
- 15. del Pilar Carrera M, Ramírez-Expósito MJ, Mayas MD, García MJ, Martínez-Martos JM. Mammary renin-angiotensin system-regulating aminopeptidase activities are modified in rats with breast cancer. Tumour Biol 31: 583–588, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol 91: 163–198, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Elased KM, Cool DR, Morris M. Novel mass spectrometric methods for evaluation of plasma angiotensin converting enzyme 1 and renin activity. Hypertension 46: 953–959, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Elased KM, Cunha TS, Gurley SB, Coffman TM, Morris M. New mass spectrometric assay for angiotensin-converting enzyme 2 activity. Hypertension 47: 1010–1017, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Fountoulakis M, Hardmeier R, Hoger H, Lubec G. Postmortem changes in the level of brain proteins. Exp Neurol 167: 86–94, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med 264: 224–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giani JF, Muñoz MC, Pons RA, Cao G, Toblli JE, Turyn D, Dominici FP. Angiotensin-(1–7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol 300: F272–F282, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 295: F772–F779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodwin RJ, Lang AM, Allingham H, Borén M, Pitt AR. Stopping the clock on proteomic degradation by heat treatment at the point of tissue excision. Proteomics 10: 1751–1761, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Goodwin RJ, Pennington SR, Pitt AR. Protein and peptides in pictures: imaging with MALDI mass spectrometry. Proteomics 8: 3785–3800, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Greis KD. Mass spectrometry for enzyme assays and inhibitor screening: an emerging application in pharmaceutical research. Mass Spectrom Rev 26: 324–339, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 116: 2218–2225, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hortin GL. The MALDI-TOF mass spectrometric view of the plasma proteome and peptidome. Clin Chem 52: 1223–1237, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Hus-Citharel A, Gasc JM, Zini S, Marchetti J, Roques B, Corvol P, Llorens-Cortes C. Aminopeptidase A activity and angiotensin III effects on [Ca2+]i along the rat nephron. Kidney Int 56: 850–859, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Iturrioz X, Rozenfeld R, Michaud A, Corvol P, Llorens-Cortes C. Study of asparagine 353 in aminopeptidase A: characterization of a novel motif (GXMEN) implicated in exopeptidase specificity of monozinc aminopeptidases. Biochemistry 40: 14440–14448, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Jones EA, van RA, van Zeijl RJ, Hogendoorn PC, Bovee JV, Deelder AM, McDonnell LA. Multiple statistical analysis techniques corroborate intratumor heterogeneity in imaging mass spectrometry datasets of myxofibrosarcoma. PLoS One 6: e24913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karamyan VT, Gembardt F, Rabey FM, Walther T, Speth RC. Characterization of the brain-specific non-AT1, non-AT2 angiotensin binding site in the mouse. Eur J Pharmacol 590: 87–92, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Khairallah PA, Bumpus FM, Page IH, Smeby RR. Angiotensinase with a high degree of specificity in plasma and red cells. Science 140: 672–674, 1963 [DOI] [PubMed] [Google Scholar]
- 34. Lapolla A, Seraglia R, Molin L, Williams K, Cosma C, Reitano R, Sechi A, Ragazzi E, Traldi P. Low molecular weight proteins in urines from healthy subjects as well as diabetic, nephropathic and diabetic-nephropathic patients: a MALDI study. J Mass Spectrom 44: 419–425, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Lejczak B, De Choszczak MP, Kafarski P. Inhibition of aminopeptidases by phosphonic acid and phosphinic acid analogues of aspartic and glutamic acids. J Enzyme Inhib 7: 97–103, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Lortie M, Bark S, Blantz R, Hook V. Detecting low-abundance vasoactive peptides in plasma: progress toward absolute quantitation using nano liquid chromatography-mass spectrometry. Anal Biochem 394: 164–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev 26: 606–643, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Monteiro MB, Senador D, Zhang WF, Morris M, Elased KM. Balance of ACE and ACE2 in diabetes: Decreased renal ACE2 activity in hypertensive db/db diabetic mice (Abstract). Hypertension 52: E91, 2008 [Google Scholar]
- 39. Nilsson A, Fehniger TE, Gustavsson L, Andersson M, Kenne K, Marko-Varga G, Andrén PE. Fine mapping the spatial distribution and concentration of unlabeled drugs within tissue micro-compartments using imaging mass spectrometry. PLoS One 5: e11411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rabey FM, Karamyan VT, Speth RC. Distribution of a novel binding site for angiotensins II and III in mouse tissues. Regul Pept 162: 5–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol 50: 112–119, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM. Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. J Am Soc Mass Spectrom 19: 1069–1077, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharman DC, Morris AD, Struthers AD. Gradual reactivation of vascular angiotensin I to angiotensin II conversion during chronic ACE inhibitor therapy in patients with diabetes mellitus. Diabetologia 50: 2061–2066, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sköld K, Svensson M, Norrman M, Sjögren B, Svenningsson P, Andrén PE. The significance of biochemical and molecular sample integrity in brain proteomics and peptidomics: stathmin 2–20 and peptides as sample quality indicators. Proteomics 7: 4445–4456, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Song L, Healy DP. Kidney aminopeptidase A and hypertension, part II: effects of angiotensin II. Hypertension 33: 746–752, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Song L, Ye M, Troyanovskaya M, Wilk E, Wilk S, Healy DP. Rat kidney glutamyl aminopeptidase (aminopeptidase A): molecular identity and cellular localization. Am J Physiol Renal Fluid Electrolyte Physiol 267: F546–F557, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med 7: 493–496, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Stoeckli M, Farmer TB, Caprioli RM. Automated mass spectrometry imaging with a matrix-assisted laser desorption ionization time-of-flight instrument. J Am Soc Mass Spectrom 10: 67–71, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Suckau D, Resemann A, Schuerenberg M, Hufnagel P, Franzen J, Holle A. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal Bioanal Chem 376: 952–965, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Tan F, Morris PW, Skidgel RA, Erdös EG. Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). Similarity to both serine carboxypeptidase and prolylendopeptidase families. J Biol Chem 268: 16631–16638, 1993 [PubMed] [Google Scholar]
- 52. Tieku S, Hooper NM. Inhibition of aminopeptidases N, A and W. A re-evaluation of the actions of bestatin and inhibitors of angiotensin converting enzyme. Biochem Pharmacol 44: 1725–1730, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Végvári A, Fehniger TE, Gustavsson L, Nilsson A, Andrén PE, Kenne K, Nilsson J, Laurell T, Marko-Varga G. Essential tactics of tissue preparation and matrix nano-spotting for successful compound imaging mass spectrometry. J Proteomics 73: 1270–1278, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Weber MA, Giles TD. Inhibiting the renin-angiotensin system to prevent cardiovascular diseases: do we need a more comprehensive strategy? Rev Cardiovasc Med 7: 45–54, 2006 [PubMed] [Google Scholar]
- 57. Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24. 11: Life Sci 52: 1461–1480, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Wilk S, Orlowski M. Inhibition of rabbit brain prolyl endopeptidase by n-benzyloxycarbonyl-prolyl-prolinal, a transition state aldehyde inhibitor. J Neurochem 41: 69–75, 1983 [DOI] [PubMed] [Google Scholar]
- 59. Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology 152: 1582–1588, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 43: 1120–1125, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci USA 93: 11968–11973, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]