Abstract
The epithelial Na+ channel (ENaC) and acid-sensitive ion channel (ASIC) branches of the ENaC/degenerin superfamily of cation channels have drawn increasing attention as potential therapeutic targets in a variety of diseases and conditions. Originally thought to be solely expressed in fluid absorptive epithelia and in neurons, it has become apparent that members of this family exhibit nearly ubiquitous expression. Therapeutic opportunities range from hypertension, due to the role of ENaC in maintaining whole body salt and water homeostasis, to anxiety disorders and pain associated with ASIC activity. As a physiologist intrigued by the fundamental mechanics of salt and water transport, it was natural that Dale Benos, to whom this series of reviews is dedicated, should have been at the forefront of research into the amiloride-sensitive sodium channel. The cloning of ENaC and subsequently the ASIC channels has revealed a far wider role for this channel family than was previously imagined. In this review, we will discuss the known and potential roles of ENaC and ASIC subunits in the wide variety of pathologies in which these channels have been implicated. Some of these, such as the role of ENaC in Liddle's syndrome are well established, others less so; however, all are related in that the fundamental defect is due to inappropriate channel activity.
Keywords: hypertension, cystic fibrosis, pain, epithelia, central nervous system, peripheral nervous system, cancer, acute respiratory distress syndrome, neurodegenerative disease, epithelial sodium channel, acid-sensitive ion channel, degenerin
the role of any ion channel in a disease process can often be distilled down into inappropriately increased or decreased activity. This translates to a problem in one of two parameters; either too many or too few channels are expressed at the cell surface (N), or the channel is open for too long or not long enough (open probability or Po). The macroscopic current (I) that is passed by a population of like channels in a cell is the product of these two numbers and the single-channel current, i(I = iNPo). Typical determinants of N include pathways that regulate trafficking of channels to and from the cell surface, the number of channels expressed in the cell, and the number of active (as opposed to silent or cryptic) channels, at the surface. Determinants of Po include posttranslational modifications, e.g., phosphorylation, mutations, or the presence of pharmacological or endogenous agonists and blockers. In some cases, a single modification can affect both N and Po, e.g., a mutation or interactions with the cytoskeleton. Multiple pathological conditions have now been associated with epithelial Na+ channels (ENaCs) and acid-sensitive ion channels (ASICs), although not all are due to channel hyper/hypo activity, but are simply the physiological response to a change in the environment, e.g., opening of ASIC channels in response to the fall in tissue pH mediating the pain caused by ischemia. Functional expression of ENaCs is also regulated by a large number of ancillary proteins such as serum and glucocorticoid kinase-1 (SGK1), a pluripotent enzyme that influences ENaC transcription and internalization (44, 238), and vasopressin, which decreases cellular retrieval (363). Several of these mechanisms have been covered in detail in a number of excellent recent reviews (59, 114, 115, 135, 164, 281, 341, 360), and only a brief outline of these different regulatory systems will be given here. In this review, we will focus on the pathologies in which ENaCs and ASICs have been directly implicated, and examine the potential for ENaC and ASIC-targeted therapeutics (see Table 1).
Table 1.
Conditions in which ENaCs and/or ASICs are potential therapeutic targets
| ENaC Subunit |
ASIC Subunit |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Therapeutic Indication | α | β | γ | δ | 1 | 2 | 3 | 4 | Reference No. |
| Angina | x | (193, 422) | |||||||
| Anxiety | x | (409, 411) | |||||||
| ARDS | x | x | x | (91, 189) | |||||
| Autoimmune encephalitis | x | (132) | |||||||
| Bladder outlet obstruction | x | x | x | (13) | |||||
| Central modulation of pain | x | x | (32, 108, 272, 347, 426) | ||||||
| Colonic carcinoma | x | x | x | (293) | |||||
| Cystic fibrosis | x | x | x | (12, 53, 61, 87, 178, 179, 228, 312) | |||||
| Deafness | x | x | x | (159, 177) | |||||
| Dental pain | x | x | (172) | ||||||
| Epilepsy | x | x | x | (45, 259, 338, 443) | |||||
| GERD | x | x | x | x | x | x | x | (2, 424) | |
| Glaucoma | x | x | x | (113) | |||||
| Glioma | x | x | x | x | (327, 396, 397) | ||||
| Huntington's disease | x | x | (415) | ||||||
| Hypertension | x | x | x | (360) | |||||
| IBD | x | x | x | x | x | (30, 267, 430, 434) | |||
| IBS | x | x | x | (295) | |||||
| Infertility | x | x | (74, 116, 229, 335) | ||||||
| Melanoma | x | x | x | (425) | |||||
| Meniere's disease | x | x | x | (220, 221, 310, 311) | |||||
| Nephropathy | x | x | (298, 299, 374) | ||||||
| Parkinson's disease | x | x | (14, 209) | ||||||
| Peripheral pain | x | x | (7, 98, 109, 214, 272, 286, 392, 399, 403) | ||||||
| Polycystic kidney disease | x | x | x | (287) | |||||
| Pulmonary infection | x | x | x | (91) | |||||
| Retinitis pigmentosa | x | x | (122, 123) | ||||||
| Salivary gland carcinoma | x | x | (428) | ||||||
| Sjogren's syndrome | x | x | x | (383) | |||||
| Stroke | x | (418–420) | |||||||
ENaC, epithelial sodium channel; ASIC, acid-sensitive ion channel; ARDS, acute respiratory distress syndrome; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Conditions Associated With ENaC Hyperactivity: Hypertension and Cystic Fibrosis
Widely regarded as a milestone in renal physiology, the cloning of ENaC by Bernard Rossier's group in the early 1990s (64, 66) has had a profound impact on cell physiology as a whole. ENaC is now widely accepted as a trimeric heteromer composed of α-, β-, and γ-subunits (151, 204, 362), and there are few tissues that do not express subunits of ENaC or one of its relations in the degenerin (Deg)/ENaC superfamily (see Fig. 1 and Table 2). Although originally expression cloned from the colon of salt-deprived rats, the significance of these studies lay in the assignment of a molecular identity to the Na+ channel of the renal collecting duct. In this location, the channel is involved in Na+ reabsorption from the tubular fluid. Although a majority (∼ 90–95%) of the daily filtered sodium load is reabsorbed in the proximal tubule and the thick ascending limb of the loop of Henle, it is ENaC that is chiefly responsible for the fine tuning of the final urinary Na+ excretion. Inappropriate elevations in this small amount of Na+ reabsorption via ENaC can lead to the development of hypertension, a major cause of mortality and morbidity in the industrialized world, with 31.3% of adults being affected in the US alone (72a).
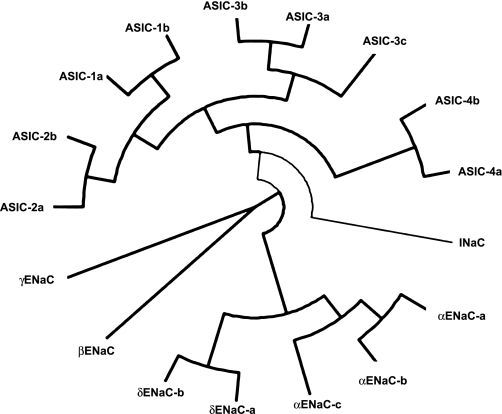
Fig. 1.
The National Center for Biotechnology Information (NCBI) reference sequences for the human epithelial Na+ channel/degenerin (ENaC/Deg) proteins were aligned in ClustalX (version 2.09) and plotted using Dendroscope (version 2.4) as a circular cladogram to illustrate the relationships between members of the hENaC and hASIC protein families. ASIC, acid-sensitive ion channel; INaC, intestinal epithelial channel.
Table 2.
Expression pattern of ASIC and ENaC subunits in different cell and tissue types
| ENaC Subunit |
ASIC Subunit |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue/Cell | α | β | γ | δ | 1 | 2 | 3 | 4 | Reference No. |
| Astrocytes | x | x | x | x | x | x | (40) | ||
| BeWo (trophoblast) | x | x | x | (96) | |||||
| Bladder | x | x | x | (13, 357) | |||||
| Brain | x | x | x | x | x | x | (6, 10, 215, 274, 307) | ||
| Chondrocytes, intervertebral discs | x | x | x | x | (203, 388, 389) | ||||
| Dorsal root ganglia | x | x | x | x | x | x | (7, 104, 186) | ||
| Esophagus | x | x | x | x | x | x | x | (2, 17, 424) | |
| Gastrointestinal tract | x | x | x | x | x | x | (64, 103, 117, 133) | ||
| Heart neurons | x | x | (167, 373) | ||||||
| Inner ear | x | x | x | x | x | (220, 221, 243, 303, 310) | |||
| Keratinocytes | x | x | x | (57) | |||||
| Kidney | x | x | x | (28) | |||||
| Lung, AT1/ ATII cells, bronchus, nasal epithelium, Calu-3 cells | x | x | x | x | x | (27, 116, 211, 212, 371) | |||
| Lymphocytes | x | x | x | (441) | |||||
| Nodose ganglia | x | x | x | x | x | (258, 372, 423) | |||
| Osteoblasts | x | x | x | x | (223) | ||||
| Pacinian corpuscles | x | x | (62) | ||||||
| Retina | x | x | x | x | x | x | x | x | (56, 149, 277) |
| Sperm | x | x | (173) | ||||||
| Taste buds | x | x | x | x | x | x | (231, 248, 250, 349) | ||
| Uterus, vagina, fallopian tube | x | x | x | (75, 116) | |||||
| Vascular smooth muscle | x | x | x | x | x | (156, 205, 206) | |||
The most common form of hypertension, essential hypertension, is a classic multifactorial disease, with both environmental and genetic factors. To date, the products of over 150 separate genes are thought to influence blood pressure regulation (281). That the activity of ENaC was a critical contributor to the development of hypertension was graphically illustrated by the linkage of Liddle's syndrome, a rare inherited disease characterized by extreme hypertension (245), to mutations in ENaC subunits (350). Ultimately, it was found that deletion of a proline/tyrosine (PY) motif in the intracellular COOH-terminal tails of β- and γENaC prevented binding of a ubiquitin ligase termed Nedd4-2 (1, 152, 165, 166, 262, 343, 361). As a consequence, the channel subunits were poorly ubiquitinated and the channel complex was not appropriately internalized, increasing channel residence time at the plasma membrane (115). This increase in N results in increased Na+ reabsorption and impressive hypertension (245). Thus the activity of Nedd4-2 is a pivotal point in ENaC regulation. Nedd4-2 can both poly- and mono-ubiquitinate ENaC, targeting ENaC for degradation by either the proteasome or lysosome, respectively (115). Activity of Nedd4-2 is, in turn, regulated by SGK1 and vasopressin; when phosphorylated by SGK1 or by PKA, Nedd4-2 is no longer able to bind, and channel is retained at the cell surface (301).
In addition to affecting channel number at the membrane, mutations can also have direct effects on channel activity causing increases in channel open probability (21, 129). Since this early work, multiple mutations have been identified in all three subunits of ENaC, some of which are associated with the development of hypertension. Whereas Liddle's syndrome was initially determined as being due to two premature stop mutations [βENaC R564stop and γENaC W574stop (165, 166, 342, 343, 350)], it is now known that point mutations in the critical proline-rich regions of the carboxyl termini, e.g., in βENaC P615H, P616R/L/S, P617L, Y618H (138, 166, 198, 213, 329, 381, 390), are additionally associated with the disease. Mutations outside this region in βENaC have also been related to inherited hypertension, e.g., E607stop (199), as have polymorphisms, e.g., T594M (26, 305, 376) although the strength of this latter linkage has been questioned (284). A more recent study has gone one step further and identified multiple single nucleotide polymorphisms in all three ENaC subunits that are associated with variations in blood pressure due to changes in dietary salt intake (440). In contrast, some mutations in ENaC are associated with the complementary autosomal recessive condition known as pseudohypoaldosteronism Type I or PHA1. As the name implies, the hallmarks of this condition are salt wasting, hypotension, and hyperkalemia and can be due to loss of function mutations in any of the three ENaC subunits (49, 77, 323, 340, 366).
However, the majority of hypertension cases have no such obvious cause as a gain of function mutation in ENaC. How then could hyperactivity of ENaC result? One way might be to increase trafficking of the channel to the membrane or to decrease its retrieval from the membrane as described above, more active surface channels resulting in increased Na+ absorption. An alternate possibility to increase the surface population of active channels could be to modify the channel on its way to the membrane or to activate silent channels already inserted into the membrane. Evidence has emerged over the past few years that ENaC undergoes a surprising degree of proteolytic processing (164, 222, 224). Initially, Vallet and colleagues showed that a serine protease called channel activating protease 1 (CAP1; prostasin), increased currents associated with ENaC in Xenopus oocyte expression systems (393, 400). Since then it has become clear that ENaC is cleaved and that two channel populations, one cleaved, one uncleaved, are trafficked to the cell membrane (224). Intracellular cleavage by furin residing in the trans-Golgi network cuts α- and γENaC, resulting in channels with substantially increased activity at the cell surface; how the uncleaved channel pool escapes this fate is currently unclear. Extracellular proteases, e.g., elastase and the channel activating proteases, also increase channel activity, and it has been proposed that this is due to the cleavage of the “silent” channel pool located at the plasma membrane (63, 187). In fact, in the absence of furin activity, channel open probability is fairly low (187, 224). Protease activity liberates endogenous inhibitory peptides from the extracellular loop of both α- and γENaC subunits (58, 67, 69, 70). Exogenous application of these inhibitory peptides to either natively expressed ENaC channels, or to channels expressed heterologously in Xenopus oocytes, was also capable of inhibiting the channel (67, 69, 297). These findings suggest novel antihypertensive therapies that include either protease inhibition or delivery of peptides to the luminal surface of the distal nephron to treat an overactive ENaC channel.
The number of channels can also be increased by affecting transcription or translation. ENaC expression is most closely associated with the mineralocorticoid, aldosterone. Serum levels of this hormone increase under conditions of sodium deprivation or potassium excess, and it is a key player in the regulation of whole body salt and water homeostasis. Aldosterone induces expression of αENaC in the collecting duct (255, 266), via a steroid response element in the 5′-flanking region of the αENaC gene (275). Both glucocorticoid and mineralocorticoid receptors can bind to this element, and both glucocorticoids and mineralocorticoids can bind the mineralocorticoid receptor (126, 134). Receptor overlap thus clearly allows for glucocorticoids to stimulate ENaC transcription. However, some degree of specificity is provided by 11-β-hydroxysteroid dehydrogenase type 2, which is highly expressed in aldosterone target tissues. This enzyme converts cortisol to cortisone (which is not a ligand for the mineralocorticoid receptor) and therefore limits the ability of glucocorticoids to activate transcription. In the kidney, the transcriptional effect of aldosterone is confined to the α- subunit; transcription of β- and γ-subunits is not affected (15, 120, 288, 365). In contrast, in the lung and colon, transcription of the β- and/or γ-subunits appears to be the rate limiting step (15, 291, 380). Additionally, ENaC transcription in the lung is under developmental control, reflecting the phenotypic switch from a secretory to an absorptive epithelium that occurs at birth (139, 170, 356).
ENaC transcription is also subject to repression, and an additional role of aldosterone is downregulation of a histone methyl-transferase termed Dot1a and its binding partner Af9. Knockout of either protein significantly increased activity of the αENaC promoter (437, 438). Serum-glucocorticoid kinase 1 (SGK1), which regulates multiple effector proteins including ENaC (95, 124, 237, 302), regulates transcription of αENaC by phosphorylating Af9, preventing the Af9/Dot1a interaction and consequently increasing αENaC expression (439); Dot1a, in turn, downregulates expression of SGK1 (320, 435), and downregulates 11-β-hydroxysteroid dehydrogenase thereby increasing the responsiveness of tissues expressing the mineralocorticoid receptor to glucocorticoids (276). There may also be a role for αENaC splice variants in the expression of the full-length ENaC subunits, as increased transcription and translation of shortened splice variants may underlie the rescue of Dahl salt-resistant rats from the deleterious consequences of a high-salt diet by interfering with normal channel assembly (344, 345). Interestingly, expression of ENaC subunits is greater in Dahl salt-sensitive rats than in the Dahl salt-resistant rats, and is further increased when these animals are placed on a high-salt diet (9, 11). Epigenetic mechanisms such as these are increasingly being appreciated as having an important role in regulation of hypertension and ENaC expression, and may well underlie differences in tissue responsiveness to aldosterone stimulation (42, 52, 435).
Perhaps the singular most defining characteristic of ENaC is its sensitivity to the diuretic amiloride. This drug blocks the channel at submicromolar (IC50 ∼0.1 μM) concentrations and is described as a K+-sparing diuretic as it inhibits ENaC-mediated Na+ absorption which reduces the driving force for K+ secretion, preventing the loss of K+ as seen with thiazide and loop diuretics. Although amiloride is highly effective, it is not widely used as a single therapy due to its potential to cause life-threatening hyperkalemia in patients with compromised ability to secrete K+; most current therapies for hypertension combine amiloride with a K+ wasting thiazide or loop diuretic or target the renin-angiotensin system and vascular smooth muscle. However, one situation in which amiloride has been proposed as an appropriate therapy is in patients with refractory hypertension. In one clinical trial, amiloride significantly reduced systolic and diastolic blood pressure (334) without causing hyperkalemia, and a case has been made that ENaC hyperactivity may underlie resistant hypertension (72, 313).
In marked contrast to hypertension, cystic fibrosis is a monogenic disease due to mutations in the cftr chloride channel (324). The classical manifestations of CF are the presence of thick sticky mucus in the airways due to poor surface hydration as a result of impaired apical Cl− secretion, a high sweat salt concentration, and poor pancreatic enzyme secretion. Experimental evidence suggests that hyperactivity of ENaC also contributes to reduced airway fluid clearance by increasing Na+ absorption and further reducing fluidity of airway mucus, a relationship first noted in CF epithelia prior to the identification of either the CFTR or ENaC genes (50, 225). An association between CFTR and ENaC was first reported by Stutts et al. (367), who showed that amiloride-sensitive Na+ transport was reduced in cells expressing both channels, whereas expression of ENaC alone was associated with large inward currents. These data suggested that wild-type CFTR exerted an inhibitory effect on ENaC to reduce ENaC Po, a finding that has been replicated by several groups (201, 241, 251, 368). The mechanism underlying this functional interaction is, however, controversial, having been variously attributed to changes in intracellular chloride affecting ENaC activity (25, 54, 230), through changes in electrical driving force (73, 181, 202), to technical issues surrounding efficiency of voltage clamping (282). CFTR and ENaC may directly interact, based on evidence obtained from planar lipid bilayer and fluorescence resonance energy transfer (FRET) studies (37, 39), and from studies demonstrating that wild-type, but not ΔF508-CFTR, protects ENaC from proteolytic cleavage, thereby limiting the number of channels in the highly active cleaved ENaC pool (147). Wild-type CFTR may also restrict the activity of ENaC by limiting ENaC expression and trafficking to the surface, an effect that was not observed in cells overexpressing ΔF508-CFTR (331). However, it has been recently reported that αENaC is expressed on the surface of motile cilia in the airways, which would suggest limited opportunities for ENaC to interact with a CFTR channel located on the apical cell membrane (116). The presence of multiple potential mechanisms likely reflects the number of different experimental models that have been adopted to address the problem, species variation, and the tendency of cells to change their repertoire of expressed proteins as they adapt to culture conditions (432). It should also be noted that sequencing of ENaC in patients with CF or unexplained CF-like disease revealed multiple mutations in all three ENaC subunits, some of which were associated with hypo- or hyperchannel activity (22, 346).
However, uncertainty as to the exact mechanisms of CFTR/ENaC interaction has not limited the exploration of ENaC as a therapeutic target in CF. Amiloride used in an aerosolized formulation in short-term clinical trials for CF improved mucociliary clearance (12, 228). However, long-term trials were disappointing, with little improvement in several parameters of lung function (312), and it became evident in the trials (61) that the rapid clearance and relatively low potency of amiloride contributed to its poor performance. Two amiloride derivatives, benzamil and phenamil, were little better, despite having improved solubility and potency (178). A third generation amiloride analog, 552-02, exhibiting greater potency, longer channel interaction time, and slower lung clearance markedly improved mucociliary clearance, but more extensive trials will need to be conducted to determine effects of this compound on lung function (179). A second ENaC-related strategy has been to use protease inhibitors in an attempt to decrease ENaC cleavage and thus reduce the potential hyperactivity (53, 87). One of these compounds, camostat, was associated with increased mucociliary clearance in sheep airways, but more extensive lung function tests have not been reported; interestingly, the effectiveness of this agent in reducing ENaC activity has led to its consideration as a novel therapy for salt-sensitive hypertension (260).
ENaC in non-CF-Related Lung Disease
Apart from regulating the depth of the airway surface liquid layer that is reduced in cystic fibrosis, the main role of sodium channels in the lung is to maintain a relatively dry environment in the alveolus to facilitate gas exchange. All three main ENaC subunits are expressed in both type I alveolar cells [AT1, involved in defense against oxidative injury (43, 212)] and type II cells [AT2, responsible for surfactant secretion (211, 380)]. There is a significant upregulation in αENaC expression in the alveoli around birth, consistent with the switch from the fluid-filled uterine to an air-breathing environment (408). Knockout studies demonstrated that mice lacking αENaC expression failed to clear the liquid that filled the lungs during gestation, dying within 40 h of birth (188). Upregulation of ENaC also provides a rationale for the treatment with corticosteroids of neonatal respiratory insufficiency seen frequently in preterm infants (29, 171, 242). Interestingly however, patients with PHA-1, who have poorly functioning ENaC, do not present with neonatal respiratory distress syndrome (RDS), but develop lung disease a few months after birth (189). Outside of the neonatal period, poorly functioning ENaC is also associated with the development of pulmonary edema (269). This condition can be caused by multiple events but is most commonly associated with increased pressure in the pulmonary vessels and/or acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Reduced ENaC activity predisposes the lung to flooding as a consequence of increased hydrostatic forces or hyper- or hypoxia (189). In contrast, mice that expressed the βENaC Liddle's mutation, i.e., a hyperactive Na+ channel, were protected from the deleterious consequences of hydrostatic edema evoked by volume overload (318). One potential therapeutic strategy therefore could be to increase activity of ENaC in the alveolar epithelium. However, although there is much experimental evidence to support increasing ENaC NPo in the alveolus using β-adrenergic receptor agonists (130, 244, 304), recent phase III trials with albuterol have been disappointing (268). One reason for this may be decreased responsiveness of alveolar β-adrenergic receptors as a consequence of cytokine release, hemorrhagic shock, and/or infection (93, 278, 386). The picture is further complicated by the presence of more than one type of amiloride-sensitive channel in alveolar epithelium. A recent study by Lazrak et al. (239) showed that two amiloride-sensitive channels, with single-channel conductances of 4.5 pS and 18 pS, could be recorded from ATI And ATII cells in lung slices from air-breathing mice. The 4.5 pS channel was highly selective for sodium and most likely corresponds to αβγENaC, while the larger channel was less sodium selective and its molecular identity is currently unknown. Interestingly, however, activity of both channels was decreased by exposure to chlorine gas, which was matched by a decrease in αENaC expression.
ENaC is also thought to have a direct role in pulmonary infections. Several bacterial and viral pathogens have been found to inhibit sodium transport in vitro, including Mycoplasma pulmonis (176, 236), Pseudomonas aeruginosa (153, 369), Mycobacterium tuberculosis (436), SARS (208), respiratory syncytial virus (RSV) (82, 90), and influenza A (235, 240), although some of the findings reported in these studies have been harder to reproduce in vivo (143, 321). Several mechanisms have been proposed to account for the effects of the pathogens. Release of nucleotides such as UTP from the invading organisms, defending immune cells, or the epithelial cells themselves would inhibit ENaC (92, 196, 197, 263, 317). Binding of UTP to specific purinergic P2Y2 receptors in the lung has been proposed to couple to the PKC pathway, PKC being a known inhibitor of ENaC activity and expression (19, 91, 364). ENaC is also inhibited by reactive oxygen and nitrogen species (RONS), e.g., peroxynitrite, released during the process of lung inflammation (111, 160, 183, 283). In contrast, superoxide seems to enhance ENaC activity, and protects against the negative influence of RONS (114, 169, 431). The potential underlying mechanisms for the negative effects of peroxynitrite include posttranslational modification of vulnerable tyrosine and/or cysteine residues in the channel, although little direct evidence has been obtained to support this (81). The effects of superoxide are most likely due to modulation of NADPH oxidase, and scavengers/inhibitors of superoxide resulted in an acute decrease in channel Po (379). This finding, which suggests that a tonic level of superoxide may be required for sustained channel activity, correlates with studies demonstrating that sustained hypoxia is associated with decreased expression of the three main ENaC subunits (384). Furthermore, pulmonary edema due to high altitude (i.e., low oxygen tension) was also associated with poor sodium transport (261). Conversely, sublethal (85%) hyperoxia increased activity of ENaC (433). Superoxide can also contribute to lung damage and the eventual failure of the respiratory epithelial barrier by reacting with NO to generate peroxynitrite (4) and nitrotyrosine, which are recovered from patients with ARDS (163), hantavirus infection, and SARS (91). Clearly, the lung must maintain a delicate balance between the positive and negative effects of free radicals to maintain an environment suitable for gas exchange—it is in cases of infection or injury that the balance tips in favor of RONS generation and decreased ENaC activity.
Other Roles for ENaC in Vascular-Cardio-Renal Disease
In addition to the contribution ENaC makes to hypertension, ENaC has also been implicated in other aspects of renal and cardiovascular disease. Several studies have suggested that ENaC subunits are expressed in vascular smooth muscle (205, 206), where they are thought to play a role in mediating myogenic constriction. While regulation is common to all vessels, it is of critical importance in the kidney, where vessel constriction in response to increases in pressure protects delicate nephrons and microvasculature from injury. Central to this response is the idea that ENaCs can act as stretch-sensitive channels, a concept originating with the finding that the ENaC family are homologs of a mechanosensitive family of proteins identified in Caenorhabditis elegans (64, 65, 264). It is tempting to speculate that the large extracellular loop characteristic of the Deg/ENaC family could be tethered to the extracellular matrix, while the cytoplasmic tails are linked to the cytoskeleton (Smith PR, unpublished observations); such an arrangement would provide a way to transduce force into channel opening (377). An alternate possibility is that direct stretch of the membrane evoked, for example, by cell swelling, could mechanically gate the channel. However, results from experiments designed to test this hypothesis have been contradictory (18, 20, 68, 207, 296). Another possibility is that flow-induced shear stress rather than membrane deformation activates the channel (5, 71), although in an oocyte-based assay it was found that shear stress did not play a part in channel activation (339), while in the intact nephron increasing flow decreased channel activity, most likely due to upstream release of inhibitory mediators (394).
The potential for a mechanosensitive role for ENaC has been most extensively investigated in the vasculature. Vascular smooth muscle cells express β- and γENaC subunits, but not αENaC (205, 206), and ENaC inhibitors, as well as knockdown of ENaC subunits, block myogenic constriction (106, 158, 205). The nature of the channel involved in this response is not entirely clear, but the lack of expression of the conducting αENaC subunit makes it unlikely that the channel involved is the “classical” αβγENaC channel of the renal collecting duct. It has been shown, that it is possible to form functional cross-clade hybrid channels composed of ENaC and ASIC subunits (273), and ASIC subunits have been identified in vascular smooth muscle cells where they play a role in cell migration (155, 156). Furthermore, myogenic constriction of the cerebellar artery was compromised in an ASIC2 knockout mouse model, suggesting that ASIC2 is intimately involved in this response (140). A more recent study has similarly proposed that an ENaC/ASIC2 hybrid may underlie mechanosensitivity in mammalian muscle spindles (352). Interestingly, a mechanosensitive role for ENaC has also been proposed in the urinary bladder, where the release of ATP from epithelial cells in response to increased hydrostatic pressure was prevented by amiloride (285). Furthermore, ENaC expression was found to be increased in the bladder mucosa patients with urinary outlet obstruction (13). Expression of βENaC also seems to be critical to the autoregulation of renal blood flow, as myogenic constriction was compromised in a murine model poorly expressing this subunit (105, 154). Thus, there is an emerging role for ENaC and the related ASIC subunits in vascular regulation.
ENaC subunits are also expressed in the nodose ganglia which receive inputs from arterial baroreceptors and it has thus been suggested that they are directly involved in sensing pressure changes transmitted from the aortic arch (107, 358), although amiloride did not block mechanosensitive currents evoked from nodose ganglia elicited by hypotonic solutions (102). ENaC may additionally play a role in the central control of blood pressure. The increase in blood pressure resulting from injections of hypertonic saline into the cerebral ventricle of rats was prevented by prior application of benzamil, suggesting that increases in sodium content of the cerebrospinal fluid may be sensed by ENaC, which is expressed in discrete locations throughout the cerebral cortex (10, 150, 378). However, here again, expression of γENaC was much more variable, suggesting that channels other than traditional αβγENaC may be involved in this response.
ENaC is also thought to be upregulated in polycystic kidney disease (PKD). The polycystic syndromes encompass a number of different genetic conditions in which the primary cilium is either shortened or absent. The most common manifestation is the development of fluid-filled cysts in the kidney and in some cases other tissues such as the liver, which is likely linked to a loss of epithelial polarity (413). PKD is associated with the development of severe hypertension; in the case of the recessive form of the disease, patients develop early and severe arterial hypertension and 20–45% will develop end-stage renal disease by their mid-teens (414). One cause of hypertension in these patients is likely due to unrestricted activity of ENaC. Studies in collecting duct cells derived from a murine genetic phenocopy model of PKD have suggested that sodium transport is upregulated, resulting in increased Na+ absorption that contributes to the development of hypertension (287). This finding would also be consistent with the low Na+ concentration of cyst fluid isolated from autosomal recessive polycystic kidney disease tissue (326).
Lastly, ENaC has been proposed to play a role in several nephropathies. Conditions such as diabetic nephropathy are associated with urinary protein loss and edema. They are also associated with increased sodium absorption in the absence of low blood volume, which would be predicted to increase sodium retention via the renin-angiotensin-aldosterone system (375). Increased proteinuria occurs due to glomerular damage, resulting in the escape of plasma protein into the tubular fluid; it can be both a consequence of, and a risk factor for, the development of hypertension. Owing to the enhancement of ENaC activity as a result of proteolytic cleavage, it has been proposed that the failure of the glomerulus to adequately remove plasma proteases from the glomerular filtrate results in increased ENaC activation with consequent increases in renal sodium absorption. One protease that has received interest in this context is plasmin, a protease cleaved from plasminogen by tissue plasminogen activator (TPA) and urokinase/u-PA. Plasmin is usually involved in fibrinolysis, providing a rationale for the use of TPA to reduce or prevent clot formation. However, levels of plasmin and plasminogen are elevated in the urine of nephrotic patients (395), while TPA and urokinase are both expressed in the kidney (233, 308). Recent evidence has shown that plasmin present in nephrotic urine can cleave γENaC, thereby activating the channel and increasing sodium absorption (298, 299, 374). Thus ENaC could be a reasonable target for therapy of nephrotic syndromes, as well as conditions such as preeclampsia (375).
ENaC in the Peripheral and Central Nervous Systems and in Sensory Perception
In addition to its role in Na+ transport in epithelia and in the vasculature, ENaC is also expressed in the nervous system and in sensory organs. Fungiform, vallate, and foliate taste buds all express ENaC subunits although expression of β and γENaC is much lower in the vallate and foliate buds (185, 231, 248, 250). This corroborated and expanded on earlier work which established that amiloride significantly attenuated the gustatory transduction of sodium (168). In mice, knocking out the conductive αENaC subunit from taste receptor cells abolished the sodium taste response (76). However, the effect of amiloride to block salt taste is not uniform, which may be explained by the presence of single nucleotide polymorphisms in the primary sequence of αENaC (127, 348). These studies suggest that a small-molecule potentiator or activator of ENaC (256) could help with adherence to a low-salt diet, by helping recreate the sensation of higher sodium intake through manipulation of the channel.
ENaC is also involved in auditory sensation where both its role in fluid transport and potential mechanosensitivity are implicated. An antibody directed against the renal sodium channel complex decorated the tips of the stereocilia of the cochlear hair cells, which is the main location for the mechano-electrical transduction or MET channel (137, 161, 162). Furthermore, amiloride blocked mechano-electrical transduction in cochlear cells in culture, and bound to tips of the cilia, suggesting that a Deg/ENaC subunit may be a component of the mechanotransducer (136, 333). However, mice in which αENaC has been knocked out exhibited no defect in hair cell mechanotransduction (332), and patients with PHA1 do not exhibit hearing loss (306), suggesting that the MET channel is not synonymous with αβγENaC.
ENaC is involved in fluid transport in the inner ear, being important for maintaining a much lower sodium concentration in the endolymph in the saccule (220, 221), and the semicircular canals (310, 311), as compared with that in the perilymph. These findings are of relevance for conditions such as Meniere's disease, where an increased volume of endolymph underlies the dysregulation of balance and symptoms of vertigo experienced by those afflicted. ENaC serves the same absorptive function in Reissner's membrane, which separates the endolymph of the scala media from the perilymph of the scala vestibuli of the cochlea (243). Interestingly, a membrane-bound serine protease that is mutated in a form of inherited deafness also activates ENaC, consistent with the experimental studies indicating a role for this channel in auditory perception (159).
The role of ENaC in other senses (vision, touch, smell), is less well established. ENaC subunits have been detected in retina and in Muller glia (55, 56, 148, 149, 277), and amiloride affects multiple components of the electroretinogram (55). In a mouse model of glaucoma, neuronal retinal expression of αENaC increased, while that of β- and γENaC decreased (113). However, the exact role of the channel in the retina requires further investigation. Both β- and γENaC (but not αENaC) have been detected in dorsal root ganglia neurons and in the specialized organs that are responsible for the detection of touch in the skin, but the exact molecular nature of the channel involved is not known (104). All three ENaC subunits have also been detected in trigeminal sensory neurons, but their exact role similarly remains unclear (131). ENaCs may be involved in sensing temperature changes, as cold was found to increase ENaC currents, but had little effect on the currents associated with ASIC expression in the absence of low pH (16). ENaCs have additionally been identified in nerve endings in the larynx and in the nodose ganglia, where they may be involved in both mechanosensitivity and chemical sensitivity detected by laryngeal taste buds (423). ENaC is also a well-established member of the channel repertoire of the nasal epithelium, but this tissue is predominantly involved in fluid transport, and is widely used in CF-related studies as a model of the airway epithelium. To date, the only member of the Deg/ENaC family that has a direct role in olfaction is the Drosophila homolog pickpocket, which is thought to be involved in sensing of female pheromones by male flies (247).
ENaC in the Gut and Reproductive Systems
The only segment of the gut proper that exhibits appreciable expression of ENaC is the distal colon. Unlike other regions in the gut, the colonic lumen contains relatively little sodium, making a diffusional pathway an appropriate mechanism for Na+ transport. As was ably demonstrated by Canessa et al. (64) in the original studies reporting the cloning of ENaC, channel expression is increased in response to low dietary sodium and is responsive to aldosterone which increases expression of the β- and γ-subunits (117, 133). Because of its location in the colon, ENaC has come under increased scrutiny as to its role in inflammatory diseases such as ulcerative colitis (125). Inflammatory mediators such as IL-1β, TNF-α, and IFN-γ downregulate expression of β- and γENaC (8, 265), reducing sodium and fluid absorption, leading to profound diarrhea. A murine model of ulcerative colitis, the IL-2 knockout mouse. also exhibited reduced expression of β- and γENaC (30). Inhibition of ENaC-mediated sodium absorption was also reported even for noninflamed colon obtained from biopsies of patients with Crohn's disease, which was attributed to TNF-α activation of the ERK pathway (434). The rotavirus enterotoxin NSP4 similarly inhibited electrogenic Na+ absorption in the colon (292). The underlying mechanism in this instance is less clear, but may involve endoplasmic reticulum calcium release with subsequent channel inhibition, or disruption of lipid rafts incorporating ENaC.
Expression of α-, β-, γ-, and δENaC subunits has also been reported in the esophagus (17, 424). Given this location, it has been proposed that the acid-sensitive δENaC may be involved in sensing low pH as experienced during heartburn (424). However, ASIC channels are also expressed in the esophagus (2), and it is likely that these channels are activated either instead of, or in addition to, ENaC. The oral mucosa also expresses an amiloride-sensitive short-circuit current that is most likely due to ENaC expression (289). In this location, ENaC activity would be associated with decreased oral hydration. This is the rationale underlying the use of the amiloride analog 552-02 in the treatment of xerostomia due to Sjogren's syndrome; the results of Phase I trials have been promising, with 552-02 improving oral hydration, speech, and the ability to eat, but the results of further trials are awaited (383).
The potential role of ENaC in the endocrine system has not been extensively studied, most work focusing on the effect of hormones on ENaC. As described above, ENaC expression and activity is tightly regulated by aldosterone, and vasopressin. However, insulin is also an important and acute regulator of ENaC activity, increasing membrane trafficking of the channel via a phosphatidylinositol 3-kinase-dependent mechanism (319, 385). It has also been suggested that growth hormone and IGF-I can increase activity of renal ENaC, which in turn contributes to the volume expansion observed in patients with acromegaly (217). Consistent with this hypothesis, it was found that ENaC activity was normalized following treatment of the underlying acromegaly (216).
Of the remaining major organ systems, the one that provides the best target for ENaC-mediated intervention is the reproductive system. Developmentally regulated amiloride-sensitive sodium transport was identified in blastocysts by Benos and collaborators in the early 1980s (35, 325), where it plays a role in the expansion of the blastocoel. Expression of all three main ENaC subunits have since been identified in a trophoblast cell line (96). ENaC is also expressed in the female reproductive tract. The main task of the tubal epithelium is maintenance of an appropriate fluid environment for sperm motility, capacitation, and, ultimately, fertilization of the oocyte. The uterine lining is also responsible for secreting fluid that is favorable for early development and ultimate embedding of the fertilized oocyte into the uterine wall. In situ hybridization studies in mice revealed that all three ENaC subunits were expressed in the surface epithelium throughout the lower part of the female genital tract (cervix, vagina), and it has recently been shown that αENaC is also expressed on the cilia lining the oviduct (75, 116). In the uterus, only αENaC was detected, and expression varied with the estrus cycle and inversely to the expression of CFTR (74). Increased fluid absorption during periods when ENaC expression is increased may be required to facilitate blastocyst implantation (335, 427). Postimplantation, uterine expression of ENaC gradually declines (74). Sperm also seem to have a basal activation of an amiloride-sensitive Na+ channel. Block of this channel resulted in hyperpolarization of the cell membrane potential, which is required for capacitation (173). These authors found that sperm expressed αENaC in the midpiece of the flagellum and δENaC in the acrosome. This latter subunit has also been identified in the testis (406); testis also expresses a unique ASIC-related member of the Deg/ENaC family (200). Although the molecular composition of the channel expressed in the reproductive tract still needs to be determined, expression of ENaC in reproductive tissues does provide a potential target for manipulation of fertility and in vitro fertilization procedures. One recent study reported that, in semen samples obtained from patients with asthenospermia, an amiloride analog (ethyl-isopropyl amiloride) caused a twofold increase in sperm motility (229).
Other Tissues
Several other tissues and cell types have also been found to express ENaC. For example, ENaC has been reported to play a role in antibody secretion from lymphocytes (441), to act as a mechanotransducer in osteoblasts (223), and to be required for correct differentiation of keratinocytes and formation of the epidermal barrier (57, 78). Whether or not ENaC can be harnessed as a drug target in these situations remains to be determined.
ASICs as Therapeutic Targets
The acid-sensitive ion channels or ASICs form a distinct branch of the Deg/ENaC superfamily of sodium channels. Four ASIC genes have been identified in humans, numbered 1–4, with a number of splice variants encoding a total of nine unique proteins (3, 24, 79, 144, 157, 253, 316, 404, 405). The channels formed by the ASIC proteins are proton-gated cation channels, opening rapidly in the presence of extracellular acid. Their fundamental role appears to be acid transduction, converting an acidic extracellular environment into a cellular signaling event, by allowing for the conduction of cations into the cell (232). In vitro, these channels activate in the pH range of 3.0–7.0, with homomeric ASIC1 channels having an EC50 at about pH 6.0 while homomeric ASIC2 channels show an EC50 at about pH 4.0 (219). Although ASIC-containing channels are primarily sodium conductors, the permeability ratio PNa+/PCa2+ for homomeric ASIC1a has been calculated to be relatively low compared with ENaC, with estimates of 2.5–18.5 (33, 405). ASICs form homomeric and heteromeric homoclade channels, all of which have distinct kinetic and activation profiles. In general, ASIC3 is the most sensitive to increases in proton concentration, with a half-maximal pH of activation of pH 6.4 (174). ASIC2 is the least sensitive, requiring exposure to pH 4.5 for half-maximal activation. ASICs have been detected throughout the nervous system, specifically in areas of high synaptic density, localizing primarily to the soma and processes of neurons (6, 219, 411). This expression pattern has suggested that these channels play a role in sensory perception and various neural processes (252, 421). However, ASICs are increasingly being detected in nonneuronal settings including cancer cells (40), bone (203), intestinal and bladder epithelium cells (103, 337), and smooth muscle (156, 226, 337).
Although the ENaC and ASIC proteins are part of the same family and have similar functions, there are considerable differences between the proteins and assembled channels. At the functional level, the primary difference is one of activation; ENaCs are constitutively active or modified by proteases to become active while ASICs are gated by a drop in extracellular pH (219). More precisely, ENaCs exhibit open and closed states, while the ASICs exhibit those states with an additional inactivated or desensitized state where the channel stops or attenuates conduction despite continued stimulation by low pH in the extracellular milieu (219, 273). This difference has consequences for channel regulation; whereas control of ENaC function is primarily determined by the number of channels at the surface, trafficking may be less critical for ASICs as the channels are to some extent ligand gated. Mutation of homoclade ASIC channels to become constitutively active leads to cell death (407). Another important difference is the presence of naturally occurring peptide toxin inhibitors of homoclade ASIC-containing channels, which are not found for homoclade ENaC-containing channels (100, 119), although there is the peptide self-inhibitory domain in some ENaC subunits that is released by proteolytic cleavage (69). ASIC1 is also subject to proteolytic cleavage, although in this case, protease activity decreases current and shifts the pH sensitivity, rather than activating the channel (85, 309, 370, 401). However, a serine protease inhibitor, nafamostat, does inhibit currents due to ASICs 1, 2, and 3 (391), although as ASICs 2 and 3 are not subject to proteolytic cleavage, the underlying mechanism is unclear. ASICs are also sensitive to amiloride and related compounds; however, the IC50 values for these drugs are around two to three orders of magnitude greater for ASIC channels as compared with the prototypical renal ENaC channel. In general, much less is known about ASIC regulation than about that for ENaC. Despite this, there are a number of therapeutic opportunities afforded by manipulation of ASIC protein function or expression, including pain states, psychiatric disorders, neurodegenerative diseases, and cancer (218, 412, 421, 428). The studies detailing the involvement of ASICs in these states come primarily from genetic knockout and inhibitor studies in animal models, though some human data are available.
ASICs and Pain
One of the chief indications for a targeted ASIC-based therapy is in the treatment of pain. The ASIC proteins as well as their characteristic acid-induced currents have been found in many cells of the peripheral nervous system (219, 412). It is also known that acidosis accompanies inflammatory and pain states, and recently a peptide toxin isolated from the Texas coral snake, the venom of which causes intense pain, has been found to be an agonist at ASIC1 and ASIC2 channels (47). These findings suggest that ASICs are involved in nociception as transducers of painful stimuli. It had been well established, with initial work from the late 1920s, that protons and acidic solutions caused painful sensations when rapidly applied (146). Identification of the ASICs and their presence in nociceptive neurons and in dorsal root ganglia rapidly led to their being considered as potential pain transducers (7, 286, 387, 399, 403). However, the interpretation of numerous studies investigating a role for ASICs in the transmission of acute cutaneous pain is complex, as there are differences between rodent models. Furthermore, knockout studies in rodents did not reveal loss of sensitivity to pain (80, 294, 314, 315), while in some cases, ASIC knockout actually increased the sensitivity to painful stimuli (80, 279). In contrast, several studies with blockers such as amiloride, its analog benzamil, and a novel non-amiloride-related compound, A-317567, have shown effects consistent with a role for ASICs; for example, the pain due to acid application or to heat could be blocked by these compounds (109, 392). Interestingly, NSAIDs such as ibuprofen and diclofenac, which seem to directly block ASICs in addition to their well-known effect on cyclooxygenase activity, also block cutaneous pain evoked by acid stimuli (214, 398). In addition to the compounds identified above, two specific peptide toxins, psalmotoxin (PcTx1), derived from a West Indian tarantula, and APETx2, derived from a sea anemone (100, 101, 118, 119), are highly specific blockers of ASIC1 and ASIC3, respectively. APETx2 is an effective blocker of pain due to arachidonic acid in rats, suggesting a role for ASIC3 in mediating pain due to inflammation. Importantly, this compound increases the pH sensitivity of ASIC3, such that this channel activates at higher pH (lower [H+]), in the presence of arachidonic acid (99). In contrast, PcTx1, which blocked pain and activated the enkephalin pathway when injected intrathecally (272), was much less effective at blocking acute pain (99); however, ASIC1 may have a role in the formation of heteromeric ASIC 1+3 channels that are not sensitive to this toxin (98).
ASICs have also been implicated in pain sensations arising from skeletal muscle and joints, heart, lung, and the gastrointestinal (GI) tract. Neurons from the dorsal root ganglia (DRG) have a central role to play in mediating pain from the joints and muscles, and the presence of ASIC expression in these tissues is highly suggestive. ASIC3 knockout mice (ASIC3−/−) did not develop hyperalgesia of the paw consequent on carrageenan-induced inflammation of the gastrocnemius muscle, although the response to heat was maintained (354, 355), most likely due to the role of TRPV channels in mediating this pain modality. Similar results have been obtained in a localized in vivo knockout mouse model (402). Furthermore, ASIC3−/− mice did not exhibit the hyperalgesia in surrounding tissues associated with joint inflammation (secondary hyperalgesia) (191). ASIC3 expression was also increased in wild-type mice following joint inflammation (191, 192). It has been suggested that the presence of channel on afferent nerves following inflammation would be relayed to the spinal cord, which would then integrate increased sensitization into the behavior characteristic of secondary hyperalgesia, and that these findings suggest a role for ASIC3 in the pain associated with arthritis (191, 192).
In the heart, ASIC3 has been associated with pain due to angina and ischemic damage following cardiac arrest. Early studies indicated that the cell bodies of neurons going to the heart via the nodose and dorsal root ganglion responded to low pH; subsequently, it was demonstrated that the kinetics of this response were similar to those seen for ASIC3 (36, 373), although more recently it has been reported that those in the DRG more closely resemble currents associated with ASIC 2a/3 heteromers (167). These responses were triggered at relatively high pH values, which can be reached relatively swiftly in the early stages of cardiac arrest (373, 422). Lactate, produced under conditions of anaerobic metabolism as found in ischemia (194), increases the sensitivity of the channel to protons, by displacing calcium bound to an external high-affinity site (195). Recently, it has been recognized that ATP, which is also released as a consequence of ischemic damage, causes a leftward shift in the response of ASIC3 to acid (46). It has been proposed that this requires protein:protein interactions between ASIC3 and a second ion channel, the ionotropic P2X5 receptor, although whether this occurs between the channels directly or through an intermediary protein remains to be determined. Since these early studies, it has been recognized that ASIC3 is also found on sensory nerve endings innervating the vasculature, where they may serve to sense muscle ischemia (280).
Unlike the relatively small changes in [H+] found in the heart, the GI tract and particularly the stomach experiences large excursions in pH during the course of the day. Gastric acid has a pH < 1 when secreted in response to a meal, although this is quickly neutralized in the gastric lumen to ∼ pH 3–4 by buffers contained in food. In pathological conditions such as duodenal or gastric ulcer, and gastroesophageal reflux disease, acid secretion can be associated with severe pain. In one study where gastric ulcers were induced in mice by short exposure of the gastric mucosa to acetic acid, gastric afferents in the DRG and nodose ganglia exhibited ASIC-like currents, which were attributed to expression of ASIC1 and ASIC2 subunits, based on the response to inhibitors (372). Signaling along the brain-gut axis was also disrupted in ASIC3 knockout mice which had been exposed to iodoacetamide to induce a mild gastritis (416). ASICs also likely contribute to the sensation of heartburn experienced by many individuals as a consequence of acid washed through the lower esophageal sphincter into the esophagus (2). ASICs 1, 2, and 3 have been detected in colonic neurons (186) and have been implicated in pain associated with inflammatory and irritable bowel syndromes. ASIC3 is upregulated in enteric neurons in the inflamed gut of patients with Crohn's disease (430), while mRNAs for ASICs 1 and 2 were upregulated in the spinal cord of a rat model of irritable bowel syndrome; furthermore, intrathecal PcTx1 prevented the development of colonic hypersensitivity in this model (267). The role of ASICs in irritable bowel syndrome may be related to a role in sensing mechanical distension throughout the gut (295), although as with studies of cutaneous pain sensation, knockout studies are somewhat equivocal (330). In addition, gut ASICs have been postulated to regulate bicarbonate secretion in the duodenum (103). ASICs 2 and 3 have also been identified in pulpal afferents and have been proposed as therapeutic targets in dentin sensitivity (172).
Although ASIC3 seems to predominate in the mediation of pain signals from the periphery, ASIC1 or composite ASIC1/2 channels may have a more important role in pain sensation in the central nervous system (CNS). ASICs are expressed in the CNS in multiple locations including hippocampus, hypothalamus, midbrain, and cerebellum (6, 215, 274, 307). While not all of these locations are involved in pain sensing, it is likely that ASICs identified in neurons of the dorsal horn are responsible for integrating peripheral pain signals before transmission to the cortex (32). The finding that PcTx1 and ASIC1 antisense oligo-deoxynucleotides infused into the intrathecal space both had analgesic effects further suggests a role for ASIC1 in the central mediation of pain (108, 272). Moreover, recently defined interactions between ASICs and peptides found within the CNS, such as the dynorphins, reinforce the possibility that CNS ASICs may be manipulated to manage pain (347, 426).
In general, and despite data from knockout animals, a reasonable case can be made for ASICs, particularly ASIC3, being attractive targets for novel analgesic strategies. Acid-induced pain in humans was attenuated by treatment with amiloride (392), suggesting that inhibitors of ASICs, potentially including peptide toxins and aminoglycoside antibiotics (145), in addition to the more conventional amiloride and NSAID analogs (128), could play a role in the treatment of pain, while avoiding the behavioral issues associated with opiates (272). A more detailed account of the literature surrounding the role of ASICs in pain is provided in an excellent recent review (98).
ASICs and Psychiatric Disorders
Studies of ASIC1 in mice have implicated it in synaptic plasticity, learning, and memory formation (409–411). In the CNS, the protein appears to localize with the postsynaptic density-95 protein (PSD-95), which likely targets ASIC1 to areas of high synaptic density (409, 410). Behavioral tests of mice overexpressing ASIC1 found increased acquired fear related behavior (411), while ASIC1 knockout mice showed a deficit in cued and contextual fear conditioning (409), suggesting that perhaps ASIC1 could play a role in anxiety or fear learning. Interestingly, it has recently been reported that decreased brain pH resulting from hypercarbia also elicits fear in mice, a behavioral response that is attenuated by ASIC1 knockout (442). However, a case-control twin study showed no association between polymorphisms of ASIC1 and anxiety spectrum disorders (175). Still, functional tests with ASIC inhibitors in animal models found that ASIC inhibition was able to produce antidepressive, sedative, and anxiolytic effects (88, 112, 234). For example, amiloride, PcTx1, and A317567 all exhibited anxiolytic activity, with PcTx1 and A317567 being most effective, most likely reflecting the lower potency of amiloride in ASIC inhibition (112). The exact role and mechanism of ASIC in anxiety and fear learning remains to be elucidated, but the data suggest that inhibition of ASICs can play a role in the treatment of anxiety or depression states.
ASICs and Neurodegenerative Disorders
ASIC proteins in the CNS have been shown to play a role in a handful of neurodegenerative disorders (421), including ischemic stroke (419), Parkinson's disease (14, 209), epilepsy (45, 259, 443), Huntington's disease (415), and autoimmune encephalitis (132). The mechanism by which ASIC proteins play a role in these pathologies varies and is still under study.
For example, during ischemic stroke there is a localized reduction in the extracellular pH. This acidosis is hypothesized to activate homomeric ASIC1 channels, leading to elevations of intracellular calcium and cell death. As noted earlier, the PNa+/PCa2+ for homotrimeric rat ASIC1a has been calculated as 2.5 by one group and as 18.5 by another group (33, 405). The divergence between these two values is likely due to the difficulties with calculating the permeability for a channel that is allosterically modulated by the divalent cation in question as well as the innate kinetics of the channel, making it difficult to record accurate measurements (23, 38, 94, 142, 195, 300). While there is debate over the PCa2+ of homotrimeric ASIC1 channels, it has been shown that cells expressing homotrimeric rat ASIC1a can respond to an acidic pH pulse with an increase in cytosolic calcium (429). This increase is due to extracellular calcium influx, as it still occurs after the endoplasmic reticulum is emptied by thapsigargin, and is sensitive to amiloride (429). This increase in calcium is postulated to lead to increased cell death secondary to acidosis; cells expressing rat ASIC1a are more sensitive to cell damage by incubation in pH 5.0 medium than cells without the ion channel as measured by a lactate dehydrogenase release assay (429). This held true for COS-7 cells exogenously expressing rat ASIC1a as well as for hippocampal neurons natively expressing rat ASICs (429). However, heteromeric channels formed of ASIC1 and ASIC2 are calcium impermeable. In rat models, global ischemia upregulated ASIC2 expression which would theoretically increase the population of heteromeric ASIC1/ASIC2 channels, acting to protect neurons from further ischemic attacks (210).
Further in vitro models, as well as in vivo mouse and rat models of stroke have shown that inhibition of ASIC1 effectively protects neurons from acidosis or ischemia (141, 353, 418–420). In vivo animal data regarding ASIC1 suggest that inhibition of this channel will be effective up to 5 h after the stroke rather than the narrow one hour window of N-methyl-d-aspartate antagonists (353). However, there is some slight controversy to this calcium-based hypothesis, as Samways et al. (336) were unable to see a significant increase in intracellular calcium in physiological conditions in a large number of cells natively or exogenously expressing ASIC1. The authors observed increases in intracellular calcium in a small population of chicken dorsal root ganglia neurons, but the majority of the chicken DRG cells and the entirety of the HEK293 and COS7 cells did not show increases in intracellular [Ca2+] when expressing chicken ASIC1 or human ASIC1b (336). The difficulty in showing significant calcium permeability of ASIC1 channels, combined with the transient and desensitizing nature of ASIC1 currents, suggests that there may be more than a calcium conductance playing a role in the neuronal death during stroke.
Another mechanism of action was postulated for the role of ASICs in Huntington's disease, where mutations in the huntingtin gene lead to the accumulation of a mutant protein with an expanded polyglutamine tract and neuronal damage (34). Studies using in vitro and in vivo models of Huntington's disease showed that the use of amiloride or short hairpin RNA directed against ASIC genes was able to reduce the impact of the disease (415). The authors showed that reducing ASIC1 or ASIC2 function or expression led to an increase in the activity of the ubiquitin-proteasome system and showed a decrease in the toxic aggregation of huntingtin-polyglutamine (415). The chemical inhibitor and ASIC1 knockdown might suggest this could be due solely to calcium conductance capabilities of ASIC1, but similar results were found with knockdown of ASIC2, which is known to form calcium-impermeable channels (219, 415). Still, this work is promising and suggests a role for ASICs in this disease and possibly other CNS diseases where protein aggregation leads to neuronal death.
Epilepsy and Parkinson's Disease
Increased activity of ASICs has been implicated in both epilepsy and Parkinson's disease. Chemically induced seizures in rats are associated with a decrease in extracellular pH (351), likely due to efflux of lactic acid into the extracellular space. This raised the possibility that ASICs could be involved during seizure activity. Using pilocarpine to induce status epilepticus, Biagini et al. (45) found that the message level for ASIC2b was quickly (within 15 min), significantly, and persistently (sustained for at least 24 h) decreased in the hippocampus. This could not be accounted for by simple neuronal death as treatment with diazepam and pentobarbital, which protect neurons, did not prevent the loss of ASIC message. ASIC1a expression was also reduced, but in a more restricted area. A more recent functional study has demonstrated that ASIC1 activity is, however, important in seizure termination, as seizures in ASIC1 knockout mice were much more severe than in their wild-type counterparts; similar results were obtained when ASIC1 was acutely inhibited in wild-type mice by intracranial injection of PcTx1 (443). Conversely, overexpression of ASIC1 shortened seizure duration. Heteromeric ASIC1a/2b channels are more sensitive to reductions in pH than either ASIC1a or ASIC2b alone (174) and reducing extracellular pH (pHo) to 6.8 was sufficient to decrease seizure activity in hippocampal slices (443). Loss of ASIC2b would therefore be predicted to decrease sensitivity of a heteromeric channel to changes in pHo. However, it is unknown whether heteromeric or homomeric channels are involved in seizure termination. Furthermore, seizure initiation and termination are highly complex processes, and the involvement of any channel type may differ based on the location of the seizure focus or even between species. Interestingly, expression of ASIC4 has been reported to be induced in pediatric patients with febrile seizures (338).
The involvement of ASICs in Parkinson's disease is a relatively recent development. Parkinson's disease is associated with neuronal degeneration, loss of dopaminergic neurons in the substantia nigra, and acidosis (51). In a mouse model of Parkinson's disease, pretreatment of animals with amiloride or crude psalmotoxin venom prevented loss of dopaminergic neurons due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration (14). The effects of amiloride were comparable to those seen for other compounds capable of preserving dopaminergic neurons in the MPTP model. These results suggest the involvement of ASIC1 in neuronal loss, but the relative nonspecificity of the agents used suggests that these data should be interpreted with caution. However, a role for ASIC2a in Parkinson's disease has emerged from studies of parkin, an E3 ubiquitin ligase that is mutated in genetically related autosomal recessive Parkinson's disease. One target of parkin appears to be PICK1 (protein interacting with C kinase 1), a protein that binds to the COOH-terminal PDZ motif of ASIC1 and ASIC2 (110, 182), and which potentiates ASIC-related currents (31, 184). Overexpression of parkin suppressed ASIC2a currents in a heterologous COS-7 cell system, while OAG (1-oleoyl-2-acetyl-sn-glycerol, a PKC activator)-stimulated currents in hippocampal neurons from a parkin knockout mouse were greatly potentiated (209). In contrast, OAG had no effect on ASIC currents recorded from wild-type mice. These data suggest that hyperactivity of ASIC contributes to neurodegeneration in Parkinson's disease.
ASICs and Sensory Perception
As with ENaCs, ASICs also have a role in sensory perception. ASICs are expressed in the retina, along with ENaCs (56, 271). Retinal ganglion cells, which integrate signals from the retina also express amiloride-sensitive proton activated currents, consistent with ASIC expression (246). Retinal neurons do experience pH fluctuations, and ASICs in this location may serve to modulate excitability. ASIC1a is found throughout the retina, including cones; inhibition with PcTx1 or knockout of ASIC1 using antisense oligonucleotides resulted in attenuation of the normal response to light as evidenced in the electroretinogram (121). In contrast, studies in ASIC2 knockout mice showed that this ASIC subunit sensitized the retina to light, and suggested that it had a negative effect on phototransduction (123). Consistent with this was the finding that ASIC2 knockout mice were more susceptible to light-induced retinal degeneration than their wild-type counterparts. ASIC3 is also important for maintaining retinal function, as knockout of this subunit similarly caused rods to die (122). Several features of the retinal damage found in these animals are similar to what is observed in human conditions such as Usher syndrome, retinitis pigmentosa, and glaucoma; whether or not there is a direct link to human disease remains to be determined. ASIC2 and ASIC3 are found in the inner ear, and although knockout animals do not exhibit obvious hearing deficits, knockout of these genes may increase susceptibility to noise (303, 330); for example, in the case of ASIC3 knockouts, hearing loss developed after the first four months of life (177). As might be predicted, ASICs are expressed in taste buds where they are, naturally enough, thought to mediate the modality of sourness (249, 254). However, there are some species differences, with mouse expressing ASICs 1 and 3, while in rat ASIC2 was also identified (254, 322, 349). However, mice are able to perceive sourness (322). Patients who could not detect sourness were also found not to express ASICs 1–3 in fungiform papilla, although, interestingly, expression of δENaC, the only acid-sensitive member of the ENaC family, was retained (190). Whether the presence of these ion channels can be exploited for the treatment of taste deficiencies remains to be seen. Touch sensation also involves ASICs, although the exact role of the different channels is still somewhat unclear. Pacinian corpuscles express ASIC1 and 2 in humans (62), although loss of ASIC1 expression did not appear to affect touch sensation in mice (294). In contrast, ASIC3 has been suggested to be the important mediator of touch sensation in mice (315).
ASICs, ENaCs, and Cancer
One of the major pathologies for which a potential role of ASICs and ENaCs is currently emerging is cancer. Multiple studies have shown that ASICs and ENaCs are associated with cell migration and proliferation, even in nontumor cells (48, 83, 97, 155, 156). ENaC expression is also upregulated in melanoma cells (425), while in salivary gland carcinoma cells, an ASIC2/3 heteromeric conductance has been proposed to underlie a proton-gated current that was not present in normal salivary gland epithelia (428). To date, the best case for involvement of ASICs and ENaCs in tumors has come from studies of colonic and brain malignancies. Using a genetic murine model of colon cancer, the heterozygous APCMin/+ mouse, Ousingsawat et al. (293) found that expression of αβγENaC was increased in the distal colon, which corresponded to an increase in sodium absorption. It was suggested that increased ENaC-mediated salt absorption would lead to decreased hydration of the colonic contents, thus leading to constipation, which has been proposed as a precipitating factor in the development of colonic carcinoma (89, 359). The increased ENaC expression and sodium absorption in this mouse were rescued by treatment with rapamycin, consistent with previous reports in lung epithelia and with the recently described role of mammalian target of rapamycin to increase ENaC currents via phosphorylation of SGK1 (227, 257, 290).
The involvement of ENaCs and ASICs in glioblastoma is somewhat more complex than their role in the previously described pathologies. Glioblastoma multiforme (GBM) are primary brain tumors of astrocytes or their progenitor cells. These tumors are characterized by high rates of invasion and dispersal throughout the brain. Tumor cells that migrate into the surrounding brain parenchyma escape surgical resection and are the source of recurrent tumors, accounting in part for the poor prognosis associated with this disease. Several members of the ASIC/ENaC family are expressed in astrocytes, cultured glioma cell lines and freshly resected tumor tissues. Messenger RNAs for ASIC1, ASIC3, γENaC, and δ-ENaC are widely expressed in these cell types, while the expression of ASIC2, ASIC4, αENaC, and βENaC are more variable (40). More importantly, constitutively active, amiloride-sensitive sodium currents were also expressed in high-grade [World Health Organization (WHO) Grades III and IV] human glioblastoma primary cultures as well as in human glioma cell lines. This conductance was not present in lower-grade gliomas or in noncancerous astrocytes (40), and exhibited selectivity for cations (but was poorly selective between cations), was inhibited by PcTx1, but was not acid gated like a homo- or heteromeric ASIC-containing channel (60). Using a strategy adopted from studies in C. elegans (180, 205), it was observed that transfection of dominant negative constructs, which decreased expression of ENaC and ASIC subunits, was able to disrupt the glioma conductance, suggesting that this was a heteroclade channel formed through interactions between αγENaC and ASIC1 proteins (218). Further studies showed that a key difference between human astrocytes and glioma cells lay in the pattern of expression of ASIC2. Although ASIC2 was expressed in normal astrocytes and low-grade gliomas, ASIC2 mRNA (and protein) was absent in ∼60% of high-grade glioma cells (40). Regardless of the presence or absence of ASIC2 mRNA, no ASIC2 protein was expressed in the plasma membrane of any glioma cell line tested (396). Electrophysiological studies of glioma cells showed that transfection of ASIC2, or increasing surface expression of ASIC2 by using glycerol and sodium phenyl-butyrate, inhibited the constitutively active, amiloride-sensitive, inward cation conductance (396). Later work showed that ASIC2 was specifically bound by Hsc70 and that knockdown of this chaperone was associated with increased surface expression of ASIC2 and loss of the constitutively activated current (397). Using an inhibitor of DNA methylation, 5-Aza-C, Xia et al. (417) were also able to increase expression of ASIC2 and rescue (i.e., inhibit) the current phenotype in those cell lines that did not express ASIC2 mRNA. Cell proliferation and migration assays carried out in these studies demonstrated that the translocation of ASIC2 to the plasma membrane inhibited the proliferation and mobility of GBM cells (396, 397). The observation that PcTx1 was a highly potent inhibitor of this conductance, and inhibited cell migration, proliferation, and volume regulation (327, 328), combined with the presence of these amiloride-sensitive currents in malignant cells and their absence in benign cells, suggests the possibility that these conductances may be useful therapeutic targets. Interestingly, several studies have shown that amiloride and its analogs reduce tumor growth and metastasis in rodent models of cancer, although the relative nonspecificity of this compound, especially at high doses where it inhibits Na+/H+ and Na+/Ca2+ exchangers and even a serine protease, makes it difficult to ascribe drug effects directly to ASIC/ENaC inhibition (see Ref. 270 for review).
Summary
As can be seen from the above discussion, ASIC and ENaC subunits are very widely expressed. In the vast majority of tissues where expression has been found, it has been accompanied by the presence of amiloride-sensitive cation currents. However, not all of these currents conform to the small-conductance, high Na+ selectivity, high amiloride-affinity fingerprint associated with expression of αβγENaC in the kidney. Similarly, not in all instances where ENaC has been identified are all three of the main subunits expressed, and in several cases, ASICs are coexpressed with ENaC subunits. While environmentally mediated increases or decreases in expression of certain subunits, e.g., the response to circulating aldosterone levels and/or posttranslational modifications such as phosphorylation or carboxymethylation, account for some of the observed differences in amiloride-sensitive sodium channel characteristics, in most cases it is unknown whether expression of another subunit or other regulatory protein would alter the current profile. In addition, some ENaC and ASIC subunits, e.g., δENaC, ASIC4, have not been well studied and the roles of these subunits are poorly defined. Expression studies, especially those based solely on RT-PCR-based amplification of mRNA, should be interpreted with caution, given the ability of the technique to identify even vanishingly small amounts of message. However, the possibility that ASIC and ENaC proteins may form cross-clade heteromers as proposed in glioma cells would significantly increase the repertoire of available current phenotypes.
Given the wide expression of these proteins and their association with multiple diseases and conditions, it is hardly surprising that they form highly attractive targets for novel therapies. It has been proposed that a constitutively active degenerin mutant of ASIC2 could be delivered by virus for expression in tumor cells, provoking unregulated influx of sodium leading to rapid cell death (382). However, small molecules offer the most attractive therapeutic options, although currently available antagonists such as amiloride, benzamil, and NSAIDS suffer from lack of specificity or efficacy or have problems associated with delivery and availability such as the peptide toxins. For example, while amiloride is highly specific for αβγENaC, it has undesirable side effects, e.g., potassium retention, which can lead to the development of severe hyperkalemia in patients with compromised renal function. In doses sufficiently high to inhibit ASIC channels, amiloride is also a reasonable blocker of plasma membrane Na+/H+ and Na+/Ca2+ exchangers. More potent amiloride analogs with reasonable binding characteristics have been developed, but the results of clinical trials are equivocal. The overarching problem, therefore, is the identification (most likely by high-throughput screening) of appropriate small molecules that combine the desirable qualities of high specificity and efficacy with limited side effects in antagonizing these channels. However, further studies to assign molecular identities to recorded current and channel characteristics of this large and diverse family will also be required.
GRANTS
Preparation of this review was supported by National Institutes of Health Grant 37206.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.J.Q. and C.M.F. prepared the figure; Y.J.Q., A.K.R., and C.M.F. drafted the manuscript; Y.J.Q., A.K.R., and C.M.F. edited and revised the manuscript; C.M.F. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. S. Matalon and M. McCarthy for critical reading of the manuscript.
Present address of Y. J. Qadri: Department of Anesthesiology, University of North Carolina, Chapel Hill, NC 27514.
REFERENCES
- 1. Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, Rotin D, Staub O. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome. J Clin Invest 103: 667–673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akiba Y, Mizumori M, Kuo M, Ham M, Guth PH, Engel E, Kaunitz JD. CO2 chemosensing in rat oesophagus. Gut 57: 1654–1664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport 11: 2217–2222, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol 106: 662–667, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol 546: 77–87, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 99: 2326–2331, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 126: 1711–1720, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Amin MS, Reza E, El-Shahat E, Wang HW, Tesson F, Leenen FH. Enhanced expression of epithelial sodium channels in the renal medulla of Dahl S rats. Can J Physiol Pharmacol 89: 159–168, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol Int 31: 1288–1291, 2007 [DOI] [PubMed] [Google Scholar]
- 12. App EM, King M, Helfesrieder R, Kohler D, Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis 141: 605–612, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Araki I, Du S, Kamiyama M, Mikami Y, Matsushita K, Komuro M, Furuya Y, Takeda M. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology 64: 1255–1260, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, Bowlby MR, Dunlop J, Monaghan M. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis 31: 334–341, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Awayda MS, Bengrine A, Tobey NA, Stockand JD, Orlando RC. Nonselective cation transport in native esophageal epithelia. Am J Physiol Cell Physiol 287: C395–C402, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Awayda MS, Ismailov, Berdiev BK, Benos DJ. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol Cell Physiol 268: C1450–C1459, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Awayda MS, Ismailov, Berdiev BK, Fuller CM, Benos DJ. Protein kinase regulation of a cloned epithelial Na+ channel. J Gen Physiol 108: 49–65, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Awayda MS, Subramanyam M. Regulation of the epithelial Na+ channel by membrane tension. J Gen Physiol 112: 97–111, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Awayda MS, Tousson A, Benos DJ. Regulation of a cloned epithelial Na+ channel by its β- and γ-subunits. Am J Physiol Cell Physiol 273: C1889–C1899, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, des Georges M, Stanke F, De Boeck K, Dupont L, Balascakova M, Hjelte L, Lebecque P, Radojkovic D, Castellani C, Schwartz M, Stuhrmann M, Schwarz M, Skalicka V, de Monestrol I, Girodon E, Ferec C, Claustres M, Tummler B, Cassiman JJ, Korbmacher C, Cuppens H. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat 30: 1093–1103, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 277: 41597–41603, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem 72: 51–57, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Bachhuber T, Konig J, Voelcker T, Murle B, Schreiber R, Kunzelmann K. Cl− interference with the epithelial Na+ channel ENaC. J Biol Chem 280: 31587–31594, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND, MacGregor GA. Association of hypertension with T594M mutation in beta subunit of epithelial sodium channels in black people resident in London. Lancet 351: 1388–1392, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel delta-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 42: 498–505, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Barbry P, Hofman P. Molecular biology of Na+ absorption. Am J Physiol Gastrointest Liver Physiol 273: G571–G585, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Barker PM, Gowen CW, Lawson EE, Knowles MR. Decreased sodium ion absorption across nasal epithelium of very premature infants with respiratory distress syndrome. J Pediatr 130: 373–377, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol 286: G244–G252, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem 277: 50463–50468, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci 28: 1498–1508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem 276: 33782–33787, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Bauer PO, Nukina N. The pathogenic mechanisms of polyglutamine diseases and current therapeutic strategies. J Neurochem 110: 1737–1765, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Benos DJ, Biggers JD, Balaban RS, Mills JW, Overstrom EW. Developmental aspects of sodium-dependent transport processes of preimplantation rabbit embryos. Soc Gen Physiol Ser 39: 211–235, 1985 [PubMed] [Google Scholar]
- 36. Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res 84: 921–928, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Berdiev BK, Cormet-Boyaka E, Tousson A, Qadri YJ, Oosterveld-Hut HM, Hong JS, Gonzales PA, Fuller CM, Sorscher EJ, Lukacs GL, Benos DJ. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem 282: 36481–36488, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Berdiev BK, Mapstone TB, Markert JM, Gillespie GY, Lockhart J, Fuller CM, Benos DJ. pH alterations “reset” Ca2+ sensitivity of brain Na+ channel 2, a degenerin/epithelial Na+ ion channel, in planar lipid bilayers. J Biol Chem 276: 38755–38761, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 5: 123–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Bergann T, Fromm A, Borden SA, Fromm M, Schulzke JD. Glucocorticoid receptor is indispensable for physiological responses to aldosterone in epithelial Na+ channel induction via the mineralocorticoid receptor in a human colonic cell line. Eur J Cell Biol 90: 432–439, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Berthiaume Y, Voisin G, Dagenais A. The alveolar type I cells: the new knight of the alveolus? J Physiol 572: 609–610, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis 8: 45–58, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sanchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 479: 410–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bondarava M, Li T, Endl E, Wehner F. Alpha-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation. Pflügers Arch 458: 675–687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonny O, Knoers N, Monnens L, Rossier BC. A novel mutation of the epithelial Na+ channel causes type 1 pseudohypoaldosteronism. Pediatr Nephrol 17: 804–808, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest 78: 1245–1252, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bowen BC, Block RE, Sanchez-Ramos J, Pattany PM, Lampman DA, Murdoch JB, Quencer RM. Proton MR spectroscopy of the brain in 14 patients with Parkinson disease. AJNR Am J Neuroradiol 16: 61–68, 1995 [PMC free article] [PubMed] [Google Scholar]
- 52. Boyd C, Naray-Fejes-Toth A. Gene regulation of ENaC subunits by serum- and glucocorticoid-inducible kinase-1. Am J Physiol Renal Physiol 288: F505–F512, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am J Physiol Lung Cell Mol Physiol 281: L16–L23, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Briel M, Greger R, Kunzelmann K. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol 508: 825–836, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brockway LM, Benos DJ, Keyser KT, Kraft TW. Blockade of amiloride-sensitive sodium channels alters multiple components of the mammalian electroretinogram. Vis Neurosci 22: 143–151, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol 283: C126–C134, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Brouard M, Casado M, Djelidi S, Barrandon Y, Farman N. Epithelial sodium channel in human epidermal keratinocytes: expression of its subunits and relation to sodium transport and differentiation. J Cell Sci 112: 3343–3352, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. J Biol Chem 285: 23527–23531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bubien JK, Ji HL, Gillespie GY, Fuller CM, Markert JM, Mapstone TB, Benos DJ. Cation selectivity and inhibition of malignant glioma Na+ channels by Psalmotoxin 1. Am J Physiol Cell Physiol 287: C1282–C1291, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Burrows E, Southern KW, Noone P. Sodium channel blockers for cystic fibrosis. Cochrane Database Syst Rev 3: CD005087, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Calavia MG, Montano JA, Garcia-Suarez O, Feito J, Guervos MA, Germana A, Del Valle M, Perez-Pinera P, Cobo J, Vega JA. Differential localization of acid-sensing ion channels 1 and 2 in human cutaneus pacinian corpuscles. Cell Mol Neurobiol 30: 841–848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 361: 467–470, 1993 [DOI] [PubMed] [Google Scholar]
- 65. Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 66. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 67. Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 283: 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carattino MD, Liu W, Hill WG, Satlin LM, Kleyman TR. Lack of a role of membrane-protein interactions in flow-dependent activation of ENaC. Am J Physiol Renal Physiol 293: F316–F324, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the α-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 294: F47–F52, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Carter AR, Zhou ZH, Calhoun DA, Bubien JK. Hyperactive ENaC identifies hypertensive individuals amenable to amiloride therapy. Am J Physiol Cell Physiol 281: C1413–C1421, 2001 [DOI] [PubMed] [Google Scholar]
- 72a. Centers for Disease Control and Prevention Health, United States, 2008. Hyattsville, MD: National Center for Health Statistics, 2008 [Google Scholar]
- 73. Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R. Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl− conductance. J Membr Biol 169: 175–188, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Chan HC, He Q, Ajonuma LC, Wang XF. Epithelial ion channels in the regulation of female reproductive tract fluid microenvironment: implications in fertility and infertility. Sheng Li Xue Bao 59: 495–504, 2007 [PubMed] [Google Scholar]
- 75. Chan LN, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, Chan HC. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J Membr Biol 185: 165–176, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 78. Charles RP, Guitard M, Leyvraz C, Breiden B, Haftek M, Haftek-Terreau Z, Stehle JC, Sandhoff K, Hummler E. Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J Biol Chem 283: 2622–2630, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen L, Fuller CM, Kleyman TR, Matalon S. Mutations in the extracellular loop of α-rENaC alter sensitivity to amiloride and reactive species. Am J Physiol Renal Physiol 286: F1202–F1208, 2004 [DOI] [PubMed] [Google Scholar]
- 82. Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 40: 588–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chifflet S, Hernandez JA, Grasso S. A possible role for membrane depolarization in epithelial wound healing. Am J Physiol Cell Physiol 288: C1420–C1430, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120: 133–145, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clark EB, Jovov B, Rooj AK, Fuller CM, Benos DJ. Proteolytic cleavage of human acid-sensing ion channel 1 by the serine protease matriptase. J Biol Chem 285: 27130–27143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Coote K, Atherton-Watson HC, Sugar R, Young A, MacKenzie-Beevor A, Gosling M, Bhalay G, Bloomfield G, Dunstan A, Bridges RJ, Sabater JR, Abraham WM, Tully D, Pacoma R, Schumacher A, Harris J, Danahay H. Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J Pharmacol Exp Ther 329: 764–774, 2009 [DOI] [PubMed] [Google Scholar]
- 88. Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci 29: 5381–5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Davies RJ, Weidema WF, Sandle GI, Palmer L, Deschner EE, DeCosse JJ. Sodium transport in a mouse model of colonic carcinogenesis. Cancer Res 47: 4646–4650, 1987 [PubMed] [Google Scholar]
- 90. Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 173: 673–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Davis IC, Matalon S. Epithelial sodium channels in the adult lung–important modulators of pulmonary health and disease. Adv Exp Med Biol 618: 127–140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 286: L112–L120, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to β-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. De Weille J, Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca(2+) ions. Brain Res 900: 277–281, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Del Monaco S, Assef Y, Kotsias BA. Epithelial sodium channel in a human trophoblast cell line (BeWo). J Membr Biol 223: 127–139, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Del Monaco SM, Marino GI, Assef YA, Damiano AE, Kotsias BA. Cell migration in BeWo cells and the role of epithelial sodium channels. J Membr Biol 232: 1–13, 2009 [DOI] [PubMed] [Google Scholar]
- 98. Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128: 549–558, 2010 [DOI] [PubMed] [Google Scholar]
- 99. Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 27: 3047–3055, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 23: 1516–1525, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon 49: 271–284, 2007 [DOI] [PubMed] [Google Scholar]
- 102. Ditting T, Linz P, Hilgers KF, Jung O, Geiger H, Veelken R. Putative role of epithelial sodium channels (ENaC) in the afferent limb of cardio renal reflexes in rats. Basic Res Cardiol 98: 388–400, 2003 [DOI] [PubMed] [Google Scholar]
- 103. Dong X, Ko KH, Chow J, Tuo B, Barrett KE, Dong H. Expression of acid-sensing ion channels in intestinal epithelial cells and their role in the regulation of duodenal mucosal bicarbonate secretion. Acta Physiol (Oxf) 201: 97–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Drummond HA, Abboud FM, Welsh MJ. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res 884: 1–12, 2000 [DOI] [PubMed] [Google Scholar]
- 105. Drummond HA, Grifoni SC, Abu-Zaid A, Gousset M, Chiposi R, Barnard JM, Murphey B, Stec DE. Renal inflammation and elevated blood pressure in a mouse model of reduced β-ENaC. Am J Physiol Renal Physiol 301: F443–F449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 107. Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998 [DOI] [PubMed] [Google Scholar]
- 108. Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci 27: 11139–11148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 110. Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 277: 5203–5208, 2002 [DOI] [PubMed] [Google Scholar]
- 111. DuVall MD, Zhu S, Fuller CM, Matalon S. Peroxynitrite inhibits amiloride-sensitive Na+ currents in Xenopus oocytes expressing αβγ-rENaC. Am J Physiol Cell Physiol 274: C1417–C1423, 1998 [DOI] [PubMed] [Google Scholar]
- 112. Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 203: 41–52, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Dyka FM, May CA, Enz R. Subunits of the epithelial sodium channel family are differentially expressed in the retina of mice with ocular hypertension. J Neurochem 94: 120–128, 2005 [DOI] [PubMed] [Google Scholar]
- 114. Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 115. Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 137: 339–353, 2012 [DOI] [PubMed] [Google Scholar]
- 117. Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol Gastrointest Liver Physiol 278: G718–G724, 2000 [DOI] [PubMed] [Google Scholar]
- 118. Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci 12: 1332–1343, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121, 2000 [DOI] [PubMed] [Google Scholar]
- 120. Escoubet B, Coureau C, Bonvalet JP, Farman N. Noncoordinate regulation of epithelial Na channel and Na pump subunit mRNAs in kidney and colon by aldosterone. Am J Physiol Cell Physiol 272: C1482–C1491, 1997 [DOI] [PubMed] [Google Scholar]
- 121. Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci 26: 5800–5809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci 50: 2417–2426, 2009 [DOI] [PubMed] [Google Scholar]
- 123. Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci 24: 1005–1012, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007 [DOI] [PubMed] [Google Scholar]
- 125. Farkas K, Yeruva S, Rakonczay Z, Jr, Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis 17: 884–898, 2011 [DOI] [PubMed] [Google Scholar]
- 126. Farman N, Rafestin-Oblin ME. Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol 280: F181–F192, 2001 [DOI] [PubMed] [Google Scholar]
- 127. Feldman GM, Mogyorosi A, Heck GL, DeSimone JA, Santos CR, Clary RA, Lyall V. Salt-evoked lingual surface potential in humans. J Neurophysiol 90: 2060–2064, 2003 [DOI] [PubMed] [Google Scholar]
- 128. Ferreira J, Santos AR, Calixto JB. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci 65: 1059–1066, 1999 [DOI] [PubMed] [Google Scholar]
- 129. Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 35: 10–19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M, von During M. Epithelial Na+ channels and stomatin are expressed in rat trigeminal mechanosensory neurons. Cell Tissue Res 299: 327–334, 2000 [DOI] [PubMed] [Google Scholar]
- 132. Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med 13: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 133. Fuller PJ, Brennan FE, Burgess JS. Acute differential regulation by corticosteroids of epithelial sodium channel subunit and Nedd4 mRNA levels in the distal colon. Pflügers Arch 441: 94–101, 2000 [DOI] [PubMed] [Google Scholar]
- 134. Fuller PJ, Lim-Tio SS, Brennan FE. Specificity in mineralocorticoid versus glucocorticoid action. Kidney Int 57: 1256–1264, 2000 [DOI] [PubMed] [Google Scholar]
- 135. Funder JW. Minireview: aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 151: 5098–5102, 2010 [DOI] [PubMed] [Google Scholar]
- 136. Furness DN, Hackney CM, Benos DJ. The binding site on cochlear stereocilia for antisera raised against renal Na+ channels is blocked by amiloride and dihydrostreptomycin. Hear Res 93: 136–146, 1996 [DOI] [PubMed] [Google Scholar]
- 137. Furness DN, Hackney CM, Evans MG. Localisation of the mechanotransducer channels in mammalian cochlear hair cells provides clues to their gating. J Physiol 588: 765–772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Furuhashi M, Kitamura K, Adachi M, Miyoshi T, Wakida N, Ura N, Shikano Y, Shinshi Y, Sakamoto K, Hayashi M, Satoh N, Nishitani T, Tomita K, Shimamoto K. Liddle's syndrome caused by a novel mutation in the proline-rich PY motif of the epithelial sodium channel beta-subunit. J Clin Endocrinol Metab 90: 340–344, 2005 [DOI] [PubMed] [Google Scholar]
- 139. Gaillard D, Hinnrasky J, Coscoy S, Hofman P, Matthay MA, Puchelle E, Barbry P. Early expression of β- and γ-subunits of epithelial sodium channel during human airway development. Am J Physiol Lung Cell Mol Physiol 278: L177–L184, 2000 [DOI] [PubMed] [Google Scholar]
- 140. Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008 [DOI] [PubMed] [Google Scholar]
- 141. Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48: 635–646, 2005 [DOI] [PubMed] [Google Scholar]
- 142. Gao J, Wu LJ, Xu L, Xu TL. Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons. Brain Res 1017: 197–207, 2004 [DOI] [PubMed] [Google Scholar]
- 143. Garat C, Rezaiguia S, Meignan M, D'Ortho MP, Harf A, Matthay MA, Jayr C. Alveolar endotoxin increases alveolar liquid clearance in rats. J Appl Physiol 79: 2021–2028, 1995 [DOI] [PubMed] [Google Scholar]
- 144. Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA 94: 1459–1464, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Garza A, Lopez-Ramirez O, Vega R, Soto E. The aminoglycosides modulate the acid-sensing ionic channel currents in dorsal root ganglion neurons from the rat. J Pharmacol Exp Ther 332: 489–499, 2010 [DOI] [PubMed] [Google Scholar]
- 146. Gaza Wv, Brandi B. Beziehungen Zwischen Wasserstoffionen-Konzentration und Schmerzempfindung. J Mol Med 5: 1123–1127, 1926 [Google Scholar]
- 147. Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem 285: 32227–32232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Golestaneh N, De Kozak Y, Klein C, Mirshahi M. Epithelial sodium channel and the mineralocorticoid receptor in cultured rat Muller glial cells. Glia 33: 160–168, 2001 [DOI] [PubMed] [Google Scholar]
- 149. Golestaneh N, Nicolas C, Picaud S, Ferrari P, Mirshahi M. The epithelial sodium channel (ENaC) in rodent retina, ontogeny and molecular identity. Curr Eye Res 21: 703–709, 2000 [PubMed] [Google Scholar]
- 150. Gomez Sanchez EP. Mineralocorticoid modulation of central control of blood pressure. Steroids 60: 69–72, 1995 [DOI] [PubMed] [Google Scholar]
- 151. Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle's syndrome. J Biol Chem 273: 30012–30017, 1998 [DOI] [PubMed] [Google Scholar]
- 153. Graham A, Steel DM, Wilson R, Cole PJ, Alton EW, Geddes DM. Effects of purified Pseudomonas rhamnolipids on bioelectric properties of sheep tracheal epithelium. Exp Lung Res 19: 77–89, 1993 [DOI] [PubMed] [Google Scholar]
- 154. Grifoni SC, Chiposi R, McKey SE, Ryan MJ, Drummond HA. Altered whole kidney blood flow autoregulation in a mouse model of reduced β-ENaC. Am J Physiol Renal Physiol 298: F285–F292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Grifoni SC, Gannon KP, Stec DE, Drummond HA. ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol 291: H3076–H3086, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport 11: 1607–1611, 2000 [DOI] [PubMed] [Google Scholar]
- 158. Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension 54: 1062–1069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, Dougherty L, Scamuffa N, Guida E, Okui M, Rossier C, Hancock M, Buchet K, Reymond A, Hummler E, Marzella PL, Kudoh J, Shimizu N, Scott HS, Antonarakis SE, Rossier BC. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet 11: 2829–2836, 2002 [DOI] [PubMed] [Google Scholar]
- 160. Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol Lung Cell Mol Physiol 274: L369–L377, 1998 [DOI] [PubMed] [Google Scholar]
- 161. Hackney CM, Furness DN, Benos DJ. Localisation of putative mechanoelectrical transducer channels in cochlear hair cells by immunoelectron microscopy. Scanning Microsc 5: 741–745; discussion 745–746, 1991 [PubMed] [Google Scholar]
- 162.Hackney CM, Furness DN, Benos DJ, Woodley JF, Barratt J. Putative immunolocalization of the mechanoelectrical transduction channels in mammalian cochlear hair cells. Proc R Soc Lond B Biol Sci 248: 215–221, 1992 [DOI] [PubMed] [Google Scholar]
- 163. Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 94: 2407–2413, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens 19: 98–105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995 [DOI] [PubMed] [Google Scholar]
- 166. Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, Nelson-Williams C, Rossier BC, Lifton RP. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci USA 92: 11495–11499, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ Res 105: 279–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984 [DOI] [PubMed] [Google Scholar]
- 169. Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Helve O, Janer C, Pitkanen O, Andersson S. Expression of the epithelial sodium channel in airway epithelium of newborn infants depends on gestational age. Pediatrics 120: 1311–1316, 2007 [DOI] [PubMed] [Google Scholar]
- 171. Helve O, Pitkanen OM, Andersson S, O'Brodovich H, Kirjavainen T, Otulakowski G. Low expression of human epithelial sodium channel in airway epithelium of preterm infants with respiratory distress. Pediatrics 113: 1267–1272, 2004 [DOI] [PubMed] [Google Scholar]
- 172. Hermanstyne TO, Markowitz K, Fan L, Gold MS. Mechanotransducers in rat pulpal afferents. J Dent Res 87: 834–838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 281: 5623–5633, 2006 [DOI] [PubMed] [Google Scholar]
- 174. Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- 175. Hettema JM, An SS, Neale MC, van den Oord EJ, Kendler KS, Chen X. Lack of association between the amiloride-sensitive cation channel 2 (ACCN2) gene and anxiety spectrum disorders. Psychiatr Genet 18: 73–79, 2008 [DOI] [PubMed] [Google Scholar]
- 176. Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hildebrand MS, de Silva MG, Klockars T, Rose E, Price M, Smith RJ, McGuirt WT, Christopoulos H, Petit C, Dahl HH. Characterisation of DRASIC in the mouse inner ear. Hear Res 190: 149–160, 2004 [DOI] [PubMed] [Google Scholar]
- 178. Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, Abraham WM, Boucher RC. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. J Pharmacol Exp Ther 311: 929–938, 2004 [DOI] [PubMed] [Google Scholar]
- 179. Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, Sabater JR, Abraham WM, Donowitz M, Cha B, Johnson KB, St George JA, Johnson MR, Boucher RC. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy) phenyl]butyl-guanidine methanesulfonate (552–02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther 325: 77–88, 2008 [DOI] [PubMed] [Google Scholar]
- 180. Hong K, Mano I, Driscoll M. In vivo structure-function analyses of Caenorhabditis elegans MEC-4, a candidate mechanosensory ion channel subunit. J Neurosci 20: 2575–2588, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Horisberger JD. ENaC-CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflügers Arch 445: 522–528, 2003 [DOI] [PubMed] [Google Scholar]
- 182. Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361: 443–450, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hu P, Ischiropoulos H, Beckman JS, Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 266: L628–L634, 1994 [DOI] [PubMed] [Google Scholar]
- 184. Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong QJ, Xie N, Long LH, Chen JG, Wang F. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol 299: C1355–C1362, 2010 [DOI] [PubMed] [Google Scholar]
- 185. Huang T, Stahler F. Effects of dietary Na+ deprivation on epithelial Na+ channel (ENaC), BDNF, and TrkB mRNA expression in the rat tongue. BMC Neurosci 10: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 500: 863–875, 2007 [DOI] [PubMed] [Google Scholar]
- 187. Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 188. Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 189. Hummler E, Planes C. Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem 25: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 190. Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One 4: e7347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137: 662–669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain 10: 336–342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Immke DC, McCleskey EW. ASIC3: a lactic acid sensor for cardiac pain. ScientificWorldJournal 1: 510–512, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001 [DOI] [PubMed] [Google Scholar]
- 195. Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 196. Inglis SK, Collett A, McAlroy HL, Wilson SM, Olver RE. Effect of luminal nucleotides on Cl− secretion and Na+ absorption in distal bronchi. Pflügers Arch 438: 621–627, 1999 [DOI] [PubMed] [Google Scholar]
- 197. Inglis SK, Olver RE, Wilson SM. Differential effects of UTP and ATP on ion transport in porcine tracheal epithelium. Br J Pharmacol 130: 367–374, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Inoue J, Iwaoka T, Tokunaga H, Takamune K, Naomi S, Araki M, Takahama K, Yamaguchi K, Tomita K. A family with Liddle's syndrome caused by a new missense mutation in the beta subunit of the epithelial sodium channel. J Clin Endocrinol Metab 83: 2210–2213, 1998 [DOI] [PubMed] [Google Scholar]
- 199. Inoue T, Okauchi Y, Matsuzaki Y, Kuwajima K, Kondo H, Horiuchi N, Nakao K, Iwata M, Yokogoshi Y, Shintani Y, Bando H, Saito S. Identification of a single cytosine base insertion mutation at Arg-597 of the beta subunit of the human epithelial sodium channel in a family with Liddle's disease. Eur J Endocrinol 138: 691–697, 1998 [DOI] [PubMed] [Google Scholar]
- 200. Ishibashi K, Marumo F. Molecular cloning of a DEG/ENaC sodium channel cDNA from human testis. Biochem Biophys Res Commun 245: 589–593, 1998 [DOI] [PubMed] [Google Scholar]
- 201. Ismailov, Berdiev BK, Shlyonsky VG, Fuller CM, Prat AG, Jovov B, Cantiello HF, Ausiello DA, Benos DJ. Role of actin in regulation of epithelial sodium channels by CFTR. Am J Physiol Cell Physiol 272: C1077–C1086, 1997 [DOI] [PubMed] [Google Scholar]
- 202. Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci USA 108: 10260–10265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun 337: 349–354, 2005 [DOI] [PubMed] [Google Scholar]
- 204. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 205. Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 206. Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005 [DOI] [PubMed] [Google Scholar]
- 207. Ji HL, Fuller CM, Benos DJ. Osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes. Am J Physiol Cell Physiol 275: C1182–C1190, 1998 [DOI] [PubMed] [Google Scholar]
- 208. Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol 296: L372–L383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Joch M, Ase AR, Chen CX, MacDonald PA, Kontogiannea M, Corera AT, Brice A, Seguela P, Fon EA. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell 18: 3105–3118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab 21: 734–740, 2001 [DOI] [PubMed] [Google Scholar]
- 211. Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 99: 1966–1971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jones ES, Owen EP, Davidson JS, Van Der Merwe L, Rayner BL. The R563Q mutation of the epithelial sodium channel beta-subunit is associated with hypertension. Cardiovasc J Afr 22: 241–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci 24: 10974–10979, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Jovov B, Tousson A, McMahon LL, Benos DJ. Immunolocalization of the acid-sensing ion channel 2a in the rat cerebellum. Histochem Cell Biol 119: 437–446, 2003 [DOI] [PubMed] [Google Scholar]
- 216. Kamenicky P, Blanchard A, Frank M, Salenave S, Letierce A, Azizi M, Lombes M, Chanson P. Body fluid expansion in acromegaly is related to enhanced epithelial sodium channel (ENaC) activity. J Clin Endocrinol Metab 96: 2127–2135, 2011 [DOI] [PubMed] [Google Scholar]
- 217. Kamenicky P, Viengchareun S, Blanchard A, Meduri G, Zizzari P, Imbert-Teboul M, Doucet A, Chanson P, Lombes M. Epithelial sodium channel is a key mediator of growth hormone-induced sodium retention in acromegaly. Endocrinology 149: 3294–3305, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 220. Kim SH, Marcus DC. Endolymphatic sodium homeostasis by extramacular epithelium of the saccule. J Neurosci 29: 15851–15858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kim SH, Marcus DC. Regulation of sodium transport in the inner ear. Hear Res 280: 21–29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kitamura K, Tomita K. Regulation of renal sodium handling through the interaction between serine proteases and serine protease inhibitors. Clin Exp Nephrol 14: 405–410, 2010 [DOI] [PubMed] [Google Scholar]
- 223. Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA 94: 1013–1018, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Knowles MR, Stutts MJ, Yankaskas JR, Gatzy JT, Boucher RC., Jr Abnormal respiratory epithelial ion transport in cystic fibrosis. Clin Chest Med 7: 285–297, 1986 [PubMed] [Google Scholar]
- 226. Kobayashi H, Yoshiyama M, Zakoji H, Takeda M, Araki I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int 104: 1746–1751, 2009 [DOI] [PubMed] [Google Scholar]
- 227. Koehl GE, Spitzner M, Ousingsawat J, Schreiber R, Geissler EK, Kunzelmann K. Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APC(Min/+) mice. Oncogene 29: 1553–1560, 2010 [DOI] [PubMed] [Google Scholar]
- 228. Kohler D, App E, Schmitz-Schumann M, Wurtemberger G, Matthys H. Inhalation of amiloride improves the mucociliary and the cough clearance in patients with cystic fibroses. Eur J Respir Dis Suppl 146: 319–326, 1986 [PubMed] [Google Scholar]
- 229. Kong XB, Ma HG, Li HG, Xiong CL. Blockade of epithelial sodium channels improves sperm motility in asthenospermia patients. Int J Androl 32: 330–336, 2009 [DOI] [PubMed] [Google Scholar]
- 230. Konig J, Schreiber R, Voelcker T, Mall M, Kunzelmann K. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl− concentration. EMBO Rep 2: 1047–1051, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47: 51–64, 1999 [DOI] [PubMed] [Google Scholar]
- 232. Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003 [DOI] [PubMed] [Google Scholar]
- 233. Kristensen P, Eriksen J, Dano K. Localization of urokinase-type plasminogen activator messenger RNA in the normal mouse by in situ hybridization. J Histochem Cytochem 39: 341–349, 1991 [DOI] [PubMed] [Google Scholar]
- 234. Kuduk SD, Di Marco CN, Chang RK, Dipardo RM, Cook SP, Cato MJ, Jovanovska A, Urban MO, Leitl M, Spencer RH, Kane SA, Bilodeau MT, Hartman GD, Bock MG. Amiloride derived inhibitors of acid-sensing ion channel-3 (ASIC3). Bioorg Med Chem Lett 19: 2514–2518, 2009 [DOI] [PubMed] [Google Scholar]
- 235. Kunzelmann K, Beesley AH, King NJ, Karupiah G, Young JA, Cook DI. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci USA 97: 10282–10287, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lambert LC, Trummell HQ, Singh A, Cassell GH, Bridges RJ. Mycoplasma pulmonis inhibits electrogenic ion transport across murine tracheal epithelial cell monolayers. Infect Immun 66: 272–279, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens 18: 439–448, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lang F, Huang DY, Vallon V. SGK, renal function and hypertension. J Nephrol 23, Suppl 16: S124–S129, 2010 [PMC free article] [PubMed] [Google Scholar]
- 239. Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR, Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine breathing mice. Am J Respir Cell Mol Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na+ channels by dexamethasone. Am J Physiol Cell Physiol 279: C762–C770, 2000 [DOI] [PubMed] [Google Scholar]
- 243. Lee JH, Marcus DC. Endolymphatic sodium homeostasis by Reissner's membrane. Neuroscience 119: 3–8, 2003 [DOI] [PubMed] [Google Scholar]
- 244. Li T, Folkesson HG. RNA interference for α-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol 290: L649–L660, 2006 [DOI] [PubMed] [Google Scholar]
- 245. Liddle GW, Bledsoe T, Coppage J, WS A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 76: 199–213, 1963 [Google Scholar]
- 246. Lilley S, LeTissier P, Robbins J. The discovery and characterization of a proton-gated sodium current in rat retinal ganglion cells. J Neurosci 24: 1013–1022, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci USA 102: 12831–12836, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999 [DOI] [PubMed] [Google Scholar]
- 249. Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J Neurophysiol 88: 133–141, 2002 [DOI] [PubMed] [Google Scholar]
- 250. Lindemann B, Barbry P, Kretz O, Bock R. Occurrence of ENaC subunit mRNA and immunocytochemistry of the channel subunits in taste buds of the rat vallate papilla. Ann NY Acad Sci 855: 116–127, 1998 [DOI] [PubMed] [Google Scholar]
- 251. Ling BN, Zuckerman JB, Lin C, Harte BJ, McNulty KA, Smith PR, Gomez LM, Worrell RT, Eaton DC, Kleyman TR. Expression of the cystic fibrosis phenotype in a renal amphibian epithelial cell line. J Biol Chem 272: 594–600, 1997 [DOI] [PubMed] [Google Scholar]
- 252. Lingueglia E. Acid sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007 [DOI] [PubMed] [Google Scholar]
- 253. Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem 272: 29778–29783, 1997 [DOI] [PubMed] [Google Scholar]
- 254. Liu L, Simon SA. Acidic stimuli activates two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res 923: 58–70, 2001 [DOI] [PubMed] [Google Scholar]
- 255. Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 256. Lu M, Echeverri F, Kalabat D, Laita B, Dahan DS, Smith RD, Xu H, Staszewski L, Yamamoto J, Ling J, Hwang N, Kimmich R, Li P, Patron E, Keung W, Patron A, Moyer BD. Small molecule activator of the human epithelial sodium channel. J Biol Chem 283: 11981–11994, 2008 [DOI] [PubMed] [Google Scholar]
- 257. Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, Shokat KM, Ashrafi K, Pearce D. mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol 21: 811–818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64: 885–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Luszczki JJ, Sawicka KM, Kozinska J, Dudra-Jastrzebska M, Czuczwar SJ. Amiloride enhances the anticonvulsant action of various antiepileptic drugs in the mouse maximal electroshock seizure model. J Neural Transm 116: 57–66, 2009 [DOI] [PubMed] [Google Scholar]
- 260. Maekawa A, Kakizoe Y, Miyoshi T, Wakida N, Ko T, Shiraishi N, Adachi M, Tomita K, Kitamura K. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. J Hypertens 27: 181–189, 2009 [DOI] [PubMed] [Google Scholar]
- 261. Mairbaurl H. Role of alveolar epithelial sodium transport in high altitude pulmonary edema (HAPE). Respir Physiol Neurobiol 151: 178–191, 2006 [DOI] [PubMed] [Google Scholar]
- 262. Malik B, Yue Q, Yue G, Chen XJ, Price SR, Mitch WE, Eaton DC. Role of Nedd4-2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits. Am J Physiol Renal Physiol 289: F107–F116, 2005 [DOI] [PubMed] [Google Scholar]
- 263. Mall M, Wissner A, Gonska T, Calenborn D, Kuehr J, Brandis M, Kunzelmann K. Inhibition of amiloride-sensitive epithelial Na(+) absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am J Respir Cell Mol Biol 23: 755–761, 2000 [DOI] [PubMed] [Google Scholar]
- 264. Mano I, Driscoll M. DEG/ENaC channels: a touchy superfamily that watches its salt. Bioessays 21: 568–578, 1999 [DOI] [PubMed] [Google Scholar]
- 265. Martinez-Augustin O, Romero-Calvo I, Suarez MD, Zarzuelo A, de Medina FS. Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis 15: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 266. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Matricon J, Gelot A, Etienne M, Lazdunski M, Muller E, Ardid D. Spinal cord plasticity and acid-sensing ion channels involvement in a rodent model of irritable bowel syndrome. Eur J Pain 15: 335–343, 2011 [DOI] [PubMed] [Google Scholar]
- 268. Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 270. Matthews H, Ranson M, Kelso MJ. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int J Cancer 129: 2051–2061, 2011 [DOI] [PubMed] [Google Scholar]
- 271. Maubaret C, Delettre C, Sola S, Hamel CP. Identification of preferentially expressed mRNAs in retina and cochlea. DNA Cell Biol 21: 781–791, 2002 [DOI] [PubMed] [Google Scholar]
- 272. Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci 10: 943–945, 2007 [DOI] [PubMed] [Google Scholar]
- 273. Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007 [DOI] [PubMed] [Google Scholar]
- 274. Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience 159: 1126–1134, 2009 [DOI] [PubMed] [Google Scholar]
- 275. Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP. The alpha-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol 15: 575–588, 2001 [DOI] [PubMed] [Google Scholar]
- 276. Millis RM. Epigenetics and hypertension. Curr Hypertens Rep 13: 21–28, 2011 [DOI] [PubMed] [Google Scholar]
- 277. Mirshahi M, Nicolas C, Mirshahi S, Golestaneh N, d'Hermies F, Agarwal MK. Immunochemical analysis of the sodium channel in rodent and human eye. Exp Eye Res 69: 21–32, 1999 [DOI] [PubMed] [Google Scholar]
- 278. Modelska K, Matthay MA, Brown LA, Deutch E, Lu LN, Pittet JF. Inhibition of β-adrenergic-dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock. Am J Physiol Lung Cell Mol Physiol 276: L844–L857, 1999 [DOI] [PubMed] [Google Scholar]
- 279. Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci 25: 9893–9901, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev 86: 709–746, 2006 [DOI] [PubMed] [Google Scholar]
- 282. Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R. CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J Physiol 564: 671–682, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Nielsen VG, Baird MS, Chen L, Matalon S. DETANONOate, a nitric oxide donor, decreases amiloride-sensitive alveolar fluid clearance in rabbits. Am J Respir Crit Care Med 161: 1154–1160, 2000 [DOI] [PubMed] [Google Scholar]
- 284. Nkeh B, Samani NJ, Badenhorst D, Libhaber E, Sareli P, Norton GR, Woodiwiss AJ. T594M variant of the epithelial sodium channel beta-subunit gene and hypertension in individuals of African ancestry in South Africa. Am J Hypertens 16: 847–852, 2003 [DOI] [PubMed] [Google Scholar]
- 285. Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39: 688–697, 2011 [DOI] [PubMed] [Google Scholar]
- 286. Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport 9: 1109–1113, 1998 [DOI] [PubMed] [Google Scholar]
- 287. Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM. Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol 290: C952–C963, 2006 [DOI] [PubMed] [Google Scholar]
- 288. Ono S, Kusano E, Muto S, Ando Y, Asano Y. A low-Na+ diet enhances expression of mRNA for epithelial Na+ channel in rat renal inner medulla. Pflügers Arch 434: 756–763, 1997 [DOI] [PubMed] [Google Scholar]
- 289. Orlando RC, Tobey NA, Schreiner VJ, Readling RD. Active electrolyte transport in mammalian buccal mucosa. Am J Physiol Gastrointest Liver Physiol 255: G286–G291, 1988 [DOI] [PubMed] [Google Scholar]
- 290. Otulakowski G, Duan W, Gandhi S, O'Brodovich H. Steroid and oxygen effects on eIF4F complex, mTOR, and ENaC translation in fetal lung epithelia. Am J Respir Cell Mol Biol 37: 457–466, 2007 [DOI] [PubMed] [Google Scholar]
- 291. Otulakowski G, Flueckiger-Staub S, Ellis L, Ramlall K, Staub O, Smith D, Durie P, O'Brodovich H. Relation between alpha, beta, and gamma human amiloride- sensitive epithelial Na+ channel mRNA levels and nasal epithelial potential difference in healthy men. Am J Respir Crit Care Med 158: 1213–1220, 1998 [DOI] [PubMed] [Google Scholar]
- 292. Ousingsawat J, Mirza M, Tian Y, Roussa E, Schreiber R, Cook DI, Kunzelmann K. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflügers Arch 461: 579–589, 2011 [DOI] [PubMed] [Google Scholar]
- 293. Ousingsawat J, Spitzner M, Schreiber R, Kunzelmann K. Upregulation of colonic ion channels in APC ( Min/+ ) mice. Pflügers Arch 456: 847–855, 2008 [DOI] [PubMed] [Google Scholar]
- 294. Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology 127: 1739–1747, 2004 [DOI] [PubMed] [Google Scholar]
- 295. Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54: 1408–1415, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol 107: 35–45, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 299: F854–F861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens 19: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem 283: 36586–36591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Paukert M, Babini E, Pusch M, Grunder S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol 124: 383–394, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Pearce D. SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem 13: 13–20, 2003 [DOI] [PubMed] [Google Scholar]
- 302. Pearce D, Kleyman TR. Salt, sodium channels, and SGK1. J Clin Invest 117: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Peng BG, Ahmad S, Chen S, Chen P, Price MP, Lin X. Acid-sensing ion channel 2 contributes a major component to acid-evoked excitatory responses in spiral ganglion neurons and plays a role in noise susceptibility of mice. J Neurosci 24: 10167–10175, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 305. Persu A, Barbry P, Bassilana F, Houot AM, Mengual R, Lazdunski M, Corvol P, Jeunemaitre X. Genetic analysis of the beta subunit of the epithelial Na+ channel in essential hypertension. Hypertension 32: 129–137, 1998 [DOI] [PubMed] [Google Scholar]
- 306. Peters TA, Levtchenko E, Cremers CW, Curfs JH, Monnens LA. No evidence of hearing loss in pseudohypoaldosteronism type 1 patients. Acta Otolaryngol (Stockh) 126: 237–239, 2006 [DOI] [PubMed] [Google Scholar]
- 307. Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci USA 103: 11376–11380, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Piedagnel R, Tiger Y, Lelongt B, Ronco PM. Urokinase (u-PA) is produced by collecting duct principal cells and is post-transcriptionally regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Cell Physiol 206: 394–401, 2006 [DOI] [PubMed] [Google Scholar]
- 309. Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem 279: 38448–38457, 2004 [DOI] [PubMed] [Google Scholar]
- 310. Pondugula SR, Raveendran NN, Ergonul Z, Deng Y, Chen J, Sanneman JD, Palmer LG, Marcus DC. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol Genomics 24: 114–123, 2006 [DOI] [PubMed] [Google Scholar]
- 311. Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol 286: F1127–F1135, 2004 [DOI] [PubMed] [Google Scholar]
- 312. Pons G, Marchand MC, d'Athis P, Sauvage E, Foucard C, Chaumet-Riffaud P, Sautegeau A, Navarro J, Lenoir G. French multicenter randomized double-blind placebo-controlled trial on nebulized amiloride in cystic fibrosis patients. The Amiloride-AFLM Collaborative Study Group. Pediatr Pulmonol 30: 25–31, 2000 [DOI] [PubMed] [Google Scholar]
- 313. Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol 16: 3154–3159, 2005 [DOI] [PubMed] [Google Scholar]
- 314. Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000 [DOI] [PubMed] [Google Scholar]
- 315. Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001 [DOI] [PubMed] [Google Scholar]
- 316. Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem 271: 7879–7882, 1996 [DOI] [PubMed] [Google Scholar]
- 317. Ramminger SJ, Collett A, Baines DL, Murphie H, McAlroy HL, Olver RE, Inglis SK, Wilson SM. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. Br J Pharmacol 128: 293–300, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Randrianarison N, Escoubet B, Ferreira C, Fontayne A, Fowler-Jaeger N, Clerici C, Hummler E, Rossier BC, Planes C. beta-Liddle mutation of the epithelial sodium channel increases alveolar fluid clearance and reduces the severity of hydrostatic pulmonary oedema in mice. J Physiol 582: 777–788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Record RD, Froelich LL, Vlahos CJ, Blazer-Yost BL. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am J Physiol Endocrinol Metab 274: E611–E617, 1998 [DOI] [PubMed] [Google Scholar]
- 320. Reisenauer MR, Wang SW, Xia Y, Zhang W. Dot1a contains three nuclear localization signals and regulates the epithelial Na+ channel (ENaC) at multiple levels. Am J Physiol Renal Physiol 299: F63–F76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, Matthay MA, Jayr C. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest 99: 325–335, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J Neurosci 24: 4088–4091, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Riepe FG, van Bemmelen MX, Cachat F, Plendl H, Gautschi I, Krone N, Holterhus PM, Theintz G, Schild L. Revealing a subclinical salt-losing phenotype in heterozygous carriers of the novel S562P mutation in the alpha subunit of the epithelial sodium channel. Clin Endocrinol (Oxf) 70: 252–258, 2009 [DOI] [PubMed] [Google Scholar]
- 324. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 325. Robinson DH, Bubien JK, Smith PR, Benos DJ. Epithelial sodium conductance in rabbit preimplantation trophectodermal cells. Dev Biol 147: 313–321, 1991 [DOI] [PubMed] [Google Scholar]
- 326. Rohatgi R, Zavilowitz B, Vergara M, Woda C, Kim P, Satlin LM. Cyst fluid composition in human autosomal recessive polycystic kidney disease. Pediatr Nephrol 20: 552–553, 2005 [DOI] [PubMed] [Google Scholar]
- 327. Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem 287: 4053–4065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Ross SB, Fuller CM, Bubien JK, Benos DJ. Amiloride-sensitive Na+ channels contribute to regulatory volume increases in human glioma cells. Am J Physiol Cell Physiol 293: C1181–C1185, 2007 [DOI] [PubMed] [Google Scholar]
- 329. Rossi E, Farnetti E, Debonneville A, Nicoli D, Grasselli C, Regolisti G, Negro A, Perazzoli F, Casali B, Mantero F, Staub O. Liddle's syndrome caused by a novel missense mutation (P617L) of the epithelial sodium channel beta subunit. J Hypertens 26: 921–927, 2008 [DOI] [PubMed] [Google Scholar]
- 330. Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol 558: 659–669, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and DeltaF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Rusch A, Hummler E. Mechano-electrical transduction in mice lacking the alpha-subunit of the epithelial sodium channel. Hear Res 131: 170–176, 1999 [DOI] [PubMed] [Google Scholar]
- 333. Rusch A, Kros CJ, Richardson GP. Block by amiloride and its derivatives of mechano-electrical transduction in outer hair cells of mouse cochlear cultures. J Physiol 474: 75–86, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, Pratt JH. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension 46: 481–487, 2005 [DOI] [PubMed] [Google Scholar]
- 335. Salleh N, Baines DL, Naftalin RJ, Milligan SR. The hormonal control of uterine luminal fluid secretion and absorption. J Membr Biol 206: 17–28, 2005 [DOI] [PubMed] [Google Scholar]
- 336. Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium 45: 319–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Sanchez-Freire V, Blanchard MG, Burkhard FC, Kessler TM, Kellenberger S, Monastyrskaya K. Acid-sensing channels in human bladder: expression, function and alterations during bladder pain syndrome. J Urol 186: 1509–1516, 2011 [DOI] [PubMed] [Google Scholar]
- 338. Sasaki K, Matsuo M, Maeda T, Zaitsu M, Hamasaki Y. Febrile seizures: characterization of double-stranded RNA-induced gene expression. Pediatr Neurol 41: 114–118, 2009 [DOI] [PubMed] [Google Scholar]
- 339. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 340. Schaedel C, Marthinsen L, Kristoffersson AC, Kornfalt R, Nilsson KO, Orlenius B, Holmberg L. Lung symptoms in pseudohypoaldosteronism type 1 are associated with deficiency of the alpha-subunit of the epithelial sodium channel. J Pediatr 135: 739–745, 1999 [DOI] [PubMed] [Google Scholar]
- 341. Schild L. The epithelial sodium channel and the control of sodium balance. Biochim Biophys Acta 1802: 1159–1165, 2010 [DOI] [PubMed] [Google Scholar]
- 342. Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci USA 92: 5699–5703, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 15: 2381–2387, 1996 [PMC free article] [PubMed] [Google Scholar]
- 344. Shehata MF. Characterization of the epithelial sodium channel alpha subunit coding and non-coding transcripts and their corresponding mRNA expression levels in Dahl R versus S rat kidney cortex on normal and high salt diet. Int Arch Med 2: 5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Shehata MF. Regulation of the epithelial sodium channel [ENaC] in kidneys of salt-sensitive Dahl rats: insights on alternative splicing. Int Arch Med 2: 28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Sheridan MB, Fong P, Groman JD, Conrad C, Flume P, Diaz R, Harris C, Knowles M, Cutting GR. Mutations in the beta-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum Mol Genet 14: 3493–3498, 2005 [DOI] [PubMed] [Google Scholar]
- 347. Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci 29: 14371–14380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Shigemura N, Ohkuri T, Sadamitsu C, Yasumatsu K, Yoshida R, Beauchamp GK, Bachmanov AA, Ninomiya Y. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC α-subunit in mice. Am J Physiol Regul Integr Comp Physiol 294: R66–R75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch Histol Cytol 69: 227–231, 2006 [DOI] [PubMed] [Google Scholar]
- 350. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 351. Siesjo BK, von Hanwehr R, Nergelius G, Nevander G, Ingvar M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab 5: 47–57, 1985 [DOI] [PubMed] [Google Scholar]
- 352. Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588: 171–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Simon RP. Acidotoxicity trumps excitotoxicity in ischemic brain. Arch Neurol 63: 1368–1371, 2006 [DOI] [PubMed] [Google Scholar]
- 354.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 355. Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 129: 102–112, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Smith DE, Otulakowski G, Yeger H, Post M, Cutz E, O'Brodovich HM. Epithelial Na(+) channel (ENaC) expression in the developing normal and abnormal human perinatal lung. Am J Respir Crit Care Med 161: 1322–1331, 2000 [DOI] [PubMed] [Google Scholar]
- 357. Smith PR, Mackler SA, Weiser PC, Brooker DR, Ahn YJ, Harte BJ, McNulty KA, Kleyman TR. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am J Physiol Renal Physiol 274: F91–F96, 1998 [DOI] [PubMed] [Google Scholar]
- 358. Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton Neurosci 98: 59–63, 2002 [DOI] [PubMed] [Google Scholar]
- 359. Sonnenberg A, Muller AD. Constipation and cathartics as risk factors of colorectal cancer: a meta-analysis. Pharmacology 47, Suppl 1: 224–233, 1993 [DOI] [PubMed] [Google Scholar]
- 360.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380, 1996 [PMC free article] [PubMed] [Google Scholar]
- 362. Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC). J Biol Chem 286: 31944–31952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Stockand JD. Vasopressin regulation of renal sodium excretion. Kidney Int 78: 849–856, 2010 [DOI] [PubMed] [Google Scholar]
- 364.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000 [DOI] [PubMed] [Google Scholar]
- 365. Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol Cell Physiol 274: C1699–C1707, 1998 [DOI] [PubMed] [Google Scholar]
- 366. Strautnieks SS, Thompson RJ, Gardiner RM, Chung E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat Genet 13: 248–250, 1996 [DOI] [PubMed] [Google Scholar]
- 367. Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850, 1995 [DOI] [PubMed] [Google Scholar]
- 368. Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 272: 14037–14040, 1997 [DOI] [PubMed] [Google Scholar]
- 369. Stutts MJ, Schwab JH, Chen MG, Knowles MR, Boucher RC. Effects of Pseudomonas aeruginosa on bronchial epithelial ion transport. Am Rev Respir Dis 134: 17–21, 1986 [DOI] [PubMed] [Google Scholar]
- 370. Su J, Tang Y, Liu L, Zhou H, Dong Q. Regulation of acid-sensing ion channel 1a function by tissue kallikrein may be through channel cleavage. Neurosci Lett 490: 46–51, 2011 [DOI] [PubMed] [Google Scholar]
- 371. Su XF, Li Q, Shrestha K, Cormet-Boyaka E, Chen L, Smith PR, Sorscher EJ, Benos DJ, Matalon S, Ji HL. Interregulation of proton-gated Na(+) channel 3 and cystic fibrosis transmembrane conductance regulator. J Biol Chem 281: 36960–36968, 2006 [DOI] [PubMed] [Google Scholar]
- 372. Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci 25: 2617–2627, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 98: 711–716, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Svenningsen P, Skott O, Jensen BL. Proteinuric diseases with sodium retention: is plasmin the link? Clin Exp Pharmacol Physiol 39: 117–124, 2012 [DOI] [PubMed] [Google Scholar]
- 376. Swift PA, MacGregor GA. The epithelial sodium channel in hypertension: genetic heterogeneity and implications for treatment with amiloride. Am J Pharmacogenomics 4: 161–168, 2004 [PubMed] [Google Scholar]
- 377. Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev 84: 1097–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 378. Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res 34: 1147–1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2− regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 298: L509–L520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Talbot CL, Bosworth DG, Briley EL, Fenstermacher DA, Boucher RC, Gabriel SE, Barker PM. Quantitation and localization of ENaC subunit expression in fetal, newborn, and adult mouse lung. Am J Respir Cell Mol Biol 20: 398–406, 1999 [DOI] [PubMed] [Google Scholar]
- 381. Tamura H, Schild L, Enomoto N, Matsui N, Marumo F, Rossier BC. Liddle disease caused by a missense mutation of beta subunit of the epithelial sodium channel gene. J Clin Invest 97: 1780–1784, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Tannous BA, Christensen AP, Pike L, Wurdinger T, Perry KF, Saydam O, Jacobs AH, Garcia-Anoveros J, Weissleder R, Sena-Esteves M, Corey DP, Breakefield XO. Mutant sodium channel for tumor therapy. Mol Ther 17: 810–819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Thelin WR, Brennan MT, Lockhart PB, Singh ML, Fox PC, Papas AS, Boucher RC. The oral mucosa as a therapeutic target for xerostomia. Oral Dis 14: 683–689, 2008 [DOI] [PubMed] [Google Scholar]
- 384. Thome UH, Davis IC, Nguyen SV, Shelton BJ, Matalon S. Modulation of sodium transport in fetal alveolar epithelial cells by oxygen and corticosterone. Am J Physiol Lung Cell Mol Physiol 284: L376–L385, 2003 [DOI] [PubMed] [Google Scholar]
- 385. Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007 [DOI] [PubMed] [Google Scholar]
- 386. Traylor ZP, Yu EN, Davis IC. Respiratory syncytial virus induces airway insensitivity to β-agonists in BALB/c mice. Am J Physiol Lung Cell Mol Physiol 298: L437–L445, 2010 [DOI] [PubMed] [Google Scholar]
- 387. Treede RD. Transduction and transmission properties of primary nociceptive afferents. Ross Fiziol Zh Im I M Sechenova 85: 205–211, 1999 [PubMed] [Google Scholar]
- 388. Trujillo E, Alvarez de la Rosa D, Mobasheri A, Gonzalez T, Canessa CM, Martin-Vasallo P. Sodium transport systems in human chondrocytes II Expression of ENaC, Na+/K+/2Cl− cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol 14: 1023–1031, 1999 [DOI] [PubMed] [Google Scholar]
- 389. Uchiyama Y, Cheng CC, Danielson KG, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J Bone Miner Res 22: 1996–2006, 2007 [DOI] [PubMed] [Google Scholar]
- 390. Uehara Y, Sasaguri M, Kinoshita A, Tsuji E, Kiyose H, Taniguchi H, Noda K, Ideishi M, Inoue J, Tomita K, Arakawa K. Genetic analysis of the epithelial sodium channel in Liddle's syndrome. J Hypertens 16: 1131–1135, 1998 [DOI] [PubMed] [Google Scholar]
- 391. Ugawa S, Ishida Y, Ueda T, Inoue K, Nagao M, Shimada S. Nafamostat mesilate reversibly blocks acid-sensing ion channel currents. Biochem Biophys Res Commun 363: 203–208, 2007 [DOI] [PubMed] [Google Scholar]
- 392. Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 110: 1185–1190, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 394. Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Vaziri ND, Gonzales EC, Shayestehfar B, Barton CH. Plasma levels and urinary excretion of fibrinolytic and protease inhibitory proteins in nephrotic syndrome. J Lab Clin Med 124: 118–124, 1994 [PubMed] [Google Scholar]
- 396. Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, Esimai O, Gillespie GY, Mapstone TB, Markert JM, Fuller CM, Bubien JK, Benos DJ. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J Biol Chem 281: 19220–19232, 2006 [DOI] [PubMed] [Google Scholar]
- 397. Vila-Carriles WH, Zhou ZH, Bubien JK, Fuller CM, Benos DJ. Participation of the chaperone Hsc70 in the trafficking and functional expression of ASIC2 in glioma cells. J Biol Chem 282: 34381–34391, 2007 [DOI] [PubMed] [Google Scholar]
- 398. Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs). Curr Drug Targets Inflamm Allergy 3: 71–79, 2004 [DOI] [PubMed] [Google Scholar]
- 399. Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol 11: 828–834, 2000 [DOI] [PubMed] [Google Scholar]
- 401. Vukicevic M, Weder G, Boillat A, Boesch A, Kellenberger S. Trypsin cleaves acid-sensing ion channel 1a in a domain that is critical for channel gating. J Biol Chem 281: 714–722, 2006 [DOI] [PubMed] [Google Scholar]
- 402. Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 152: 2348–2356, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Waldmann R. Proton-gated cation channels–neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol 502: 293–304, 2001 [DOI] [PubMed] [Google Scholar]
- 404. Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997 [DOI] [PubMed] [Google Scholar]
- 405. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 406. Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995 [DOI] [PubMed] [Google Scholar]
- 407. Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem 271: 10433–10436, 1996 [DOI] [PubMed] [Google Scholar]
- 408. Watanabe S, Matsushita K, Stokes JB, McCray PB., Jr Developmental regulation of epithelial sodium channel subunit mRNA expression in rat colon and lung. Am J Physiol Gastrointest Liver Physiol 275: G1227–G1235, 1998 [DOI] [PubMed] [Google Scholar]
- 409. Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 23: 5496–5502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002 [DOI] [PubMed] [Google Scholar]
- 411. Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci USA 101: 3621–3626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 413. Wilson PD. Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta 1812: 1239–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 414. Wilson PD. Mouse models of polycystic kidney disease. Curr Top Dev Biol 84: 311–350, 2008 [DOI] [PubMed] [Google Scholar]
- 415.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, Tosaki A, Yamada M, Knopfel T, Nakamura T, Nukina N. Blocking acid-sensing ion channel 1 alleviates Huntington's disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet 17: 3223–3235, 2008 [DOI] [PubMed] [Google Scholar]
- 416. Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Lazdunski M, Waldmann R, Holzer P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain 134: 245–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Xia J, Zhou ZH, Bubien JK, Fuller CM, Markert JM, Mapstone TB, Yancey Gillespie G, Benos DJ. Molecular cloning and characterization of human acid sensing ion channel (ASIC)2 gene promoter. Gene 313: 91–101, 2003 [DOI] [PubMed] [Google Scholar]
- 418. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 419. Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels–novel therapeutic targets for ischemic brain injury. Front Biosci 12: 1376–1386, 2007 [DOI] [PubMed] [Google Scholar]
- 420. Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 209: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 421. Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol 8: 25–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509, 2006 [DOI] [PubMed] [Google Scholar]
- 423. Yamamoto Y, Taniguchi K. Expression of ENaC subunits in sensory nerve endings in the rat larynx. Neurosci Lett 402: 227–232, 2006 [DOI] [PubMed] [Google Scholar]
- 424.Yamamura H, Ugawa S, Ueda T, Nagao M, Joh T, Shimada S. Epithelial Na+ channel delta subunit is an acid sensor in the human oesophagus. Eur J Pharmacol 600: 32–36, 2008 [DOI] [PubMed] [Google Scholar]
- 425. Yamamura H, Ugawa S, Ueda T, Shimada S. Expression analysis of the epithelial Na+ channel delta subunit in human melanoma G-361 cells. Biochem Biophys Res Commun 366: 489–492, 2008 [DOI] [PubMed] [Google Scholar]
- 426. Yang HY, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides 42: 1–18, 2008 [DOI] [PubMed] [Google Scholar]
- 427. Yang JZ, Ajonuma LC, Tsang LL, Lam SY, Rowlands DK, Ho LS, Zhou CX, Chung YW, Chan HC. Differential expression and localization of CFTR and ENaC in mouse endometrium during pre-implantation. Cell Biol Int 28: 433–439, 2004 [DOI] [PubMed] [Google Scholar]
- 428. Ye JH, Gao J, Wu YN, Hu YJ, Zhang CP, Xu TL. Identification of acid-sensing ion channels in adenoid cystic carcinomas. Biochem Biophys Res Commun 355: 986–992, 2007 [DOI] [PubMed] [Google Scholar]
- 429. Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101: 6752–6757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Yiangou Y, Facer P, Smith JA, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur J Gastroenterol Hepatol 13: 891–896, 2001 [DOI] [PubMed] [Google Scholar]
- 431. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]
- 432. Yue G, Hu P, Oh Y, Jilling T, Shoemaker RL, Benos DJ, Cragoe EJ, Jr, Matalon S. Culture-induced alterations in alveolar type II cell Na+ conductance. Am J Physiol Cell Physiol 265: C630–C640, 1993 [DOI] [PubMed] [Google Scholar]
- 433. Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matalon S. Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats. Proc Natl Acad Sci USA 92: 8418–8422, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Zeissig S, Bergann T, Fromm A, Bojarski C, Heller F, Guenther U, Zeitz M, Fromm M, Schulzke JD. Altered ENaC expression leads to impaired sodium absorption in the noninflamed intestine in Crohn's disease. Gastroenterology 134: 1436–1447, 2008 [DOI] [PubMed] [Google Scholar]
- 435. Zhang D, Yu ZY, Cruz P, Kong Q, Li S, Kone BC. Epigenetics and the control of epithelial sodium channel expression in collecting duct. Kidney Int 75: 260–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Zhang M, Kim KJ, Iyer D, Lin Y, Belisle J, McEnery K, Crandall ED, Barnes PF. Effects of Mycobacterium tuberculosis on the bioelectric properties of the alveolar epithelium. Infect Immun 65: 692–698, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Zhang W, Xia X, Jalal DI, Kuncewicz T, Xu W, Lesage GD, Kone BC. Aldosterone-sensitive repression of ENaCα transcription by a histone H3 lysine-79 methyltransferase. Am J Physiol Cell Physiol 290: C936–C946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem 281: 18059–18068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Zhao Q, Gu D, Hixson JE, Liu DP, Rao DC, Jaquish CE, Kelly TN, Lu F, Ma J, Mu J, Shimmin LC, Chen J, Mei H, Hamm LL, He J. Common variants in epithelial sodium channel genes contribute to salt sensitivity of blood pressure: The GenSalt study. Circ Cardiovasc Genet 4: 375–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Zhou ZH, Bubien JK. ENaC plays a role in regulated antibody secretion by hybridomas. Am J Physiol Cell Physiol 283: C1480–C1491, 2002 [DOI] [PubMed] [Google Scholar]
- 442. Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139: 1012–1021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 11: 816–822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]