Abstract
The acute effect of i.v. and direct intrarenal arterial infusion of 25-hydroxycholecalciferol (25HCC) and 1,25-dihydroxycholecalciferol (1,25-DHCC) on renal handling of phosphorus was evaluated in the following groups of rats: (a) intact animals, (b) parathyroidectomized (PTX) hypocalcemic rats, (c) PTX rats in which normocalcemia was maintained with calcium supplements and (d) PTX animals in which urinary phosphorus was augmented by (i) i.v. sodium phosphate, (ii) expansion of the extracellular fluid volume with normal saline, and (iii) i.v. parathyroid hormone (PTH). Clearances of inulin (CIn), phosphorus (CP), and fractional clearances of phosphorus (CP/CIn) of the experimental groups were compared with those of the corresponding control groups, and the clearances of the infused kidneys with those of the contralateral kidneys.
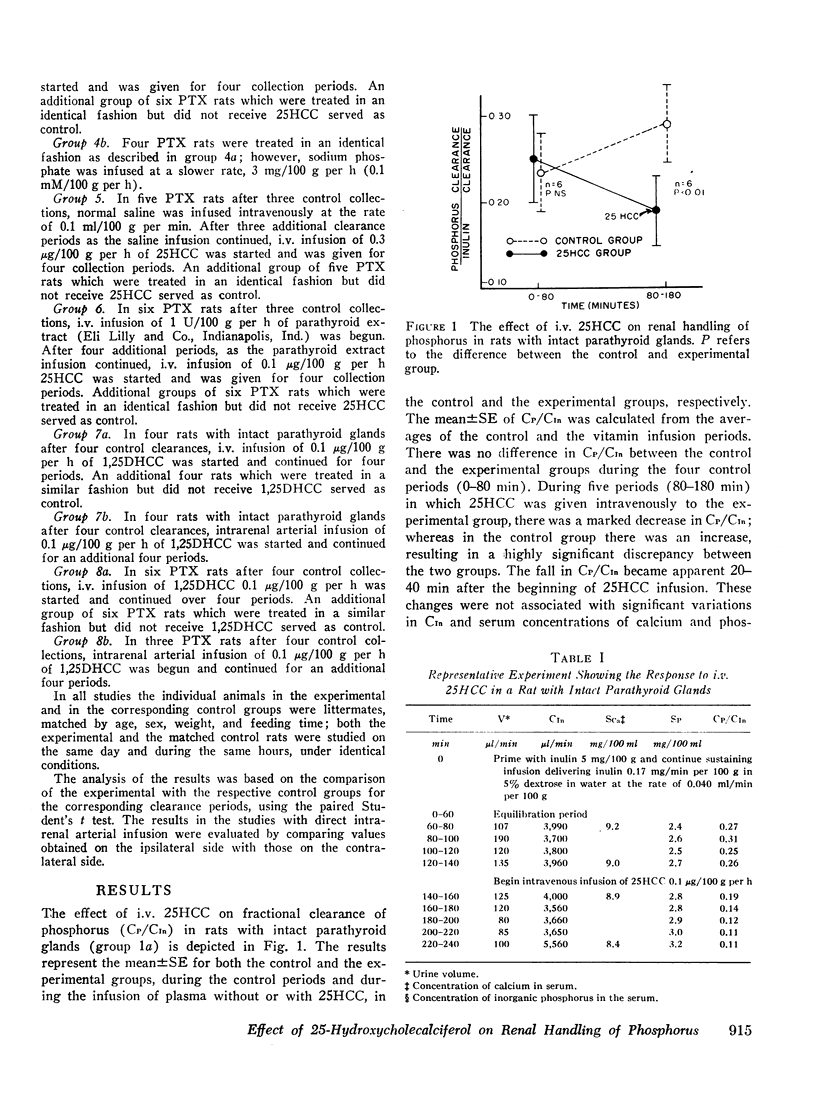
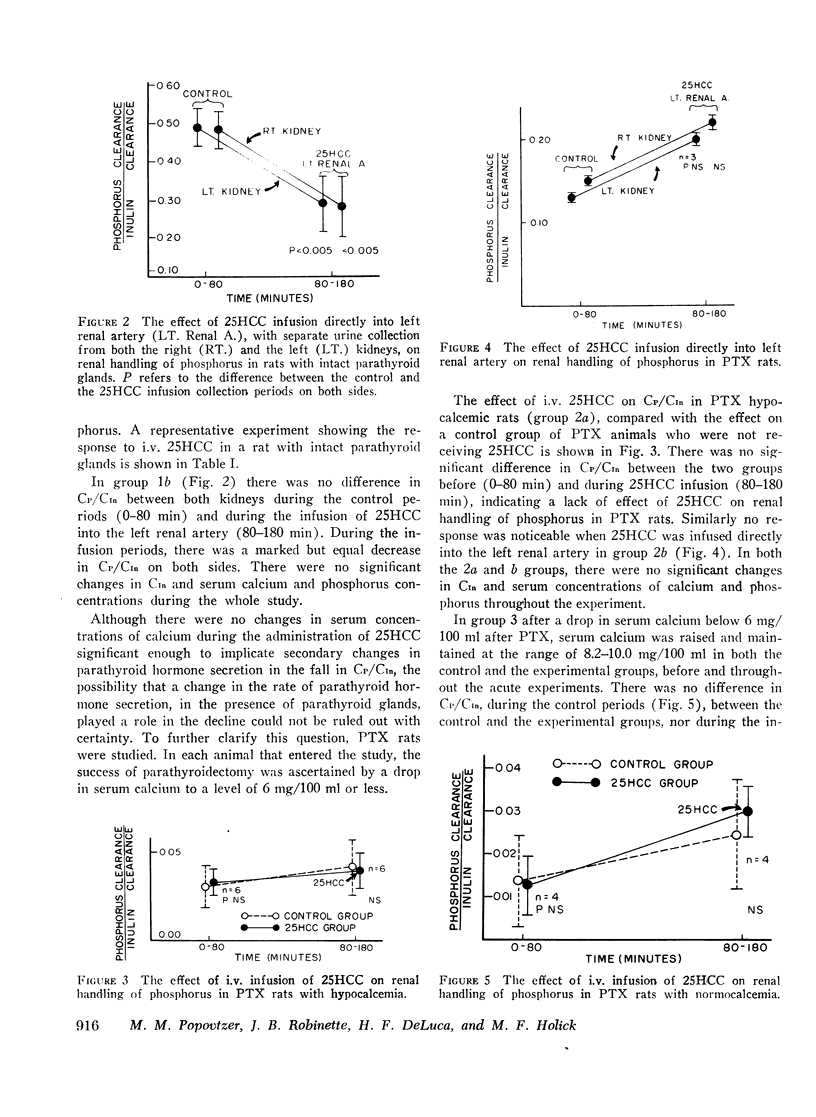
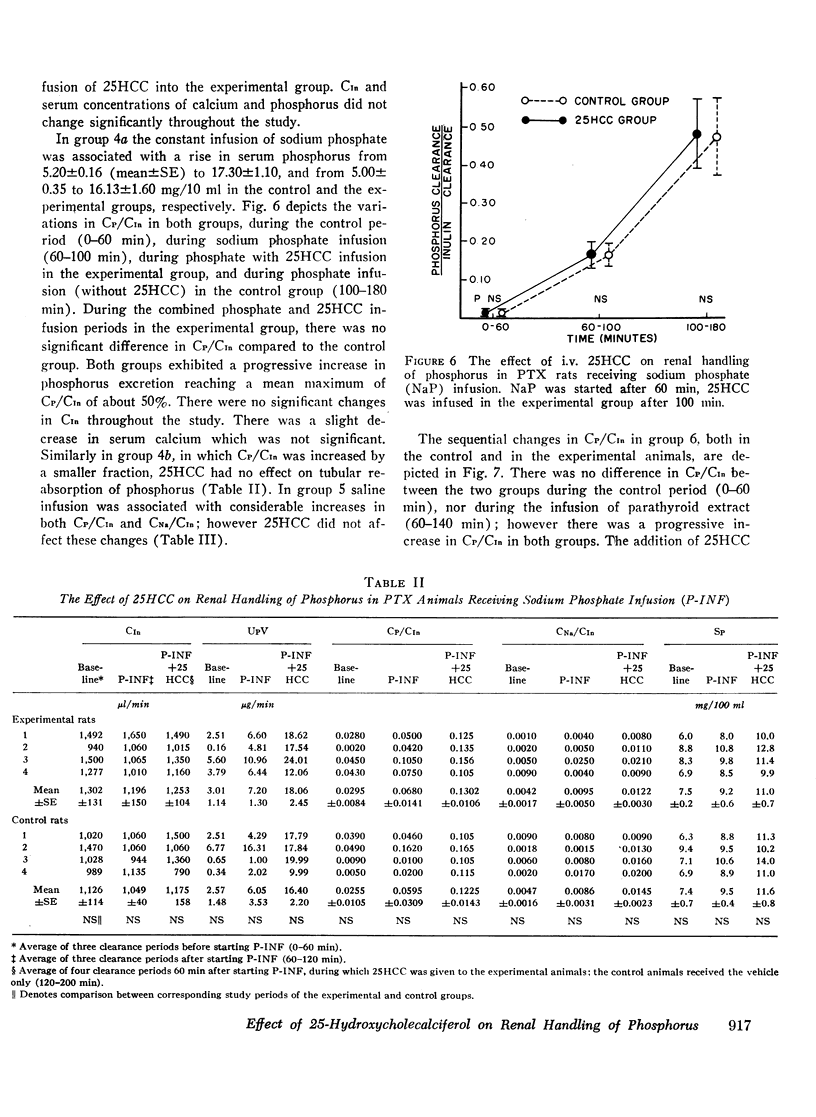
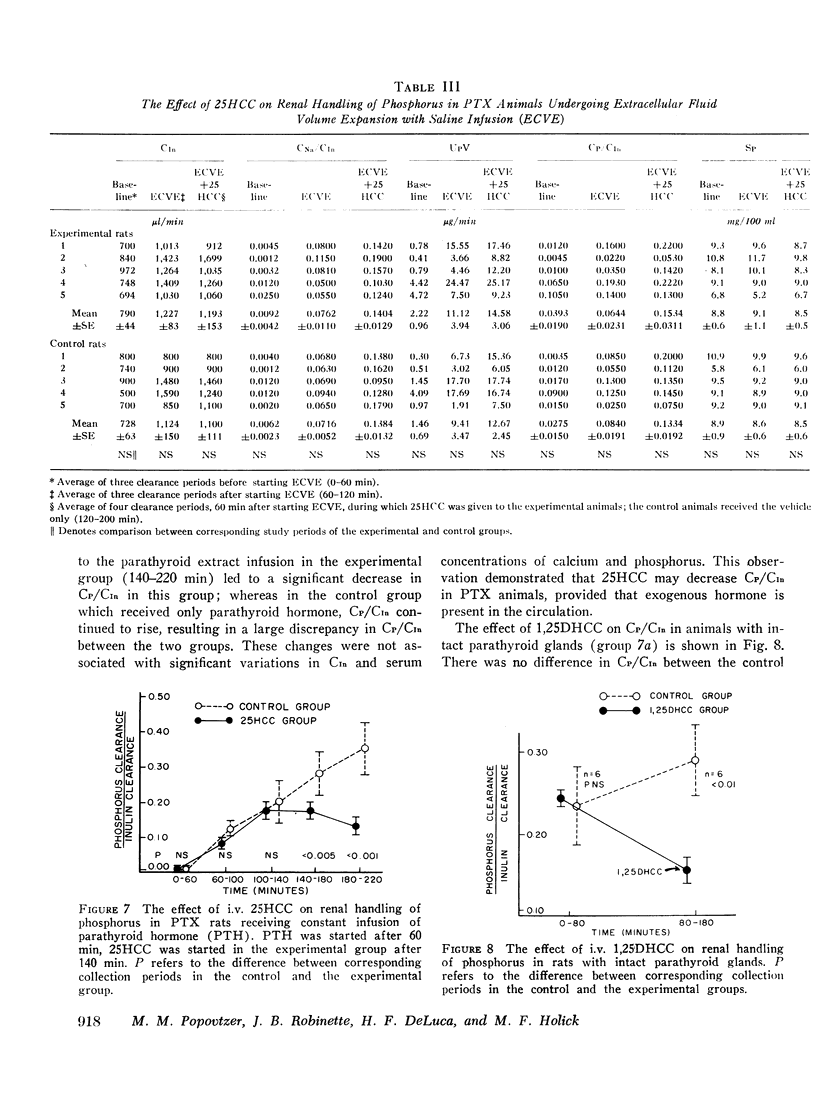
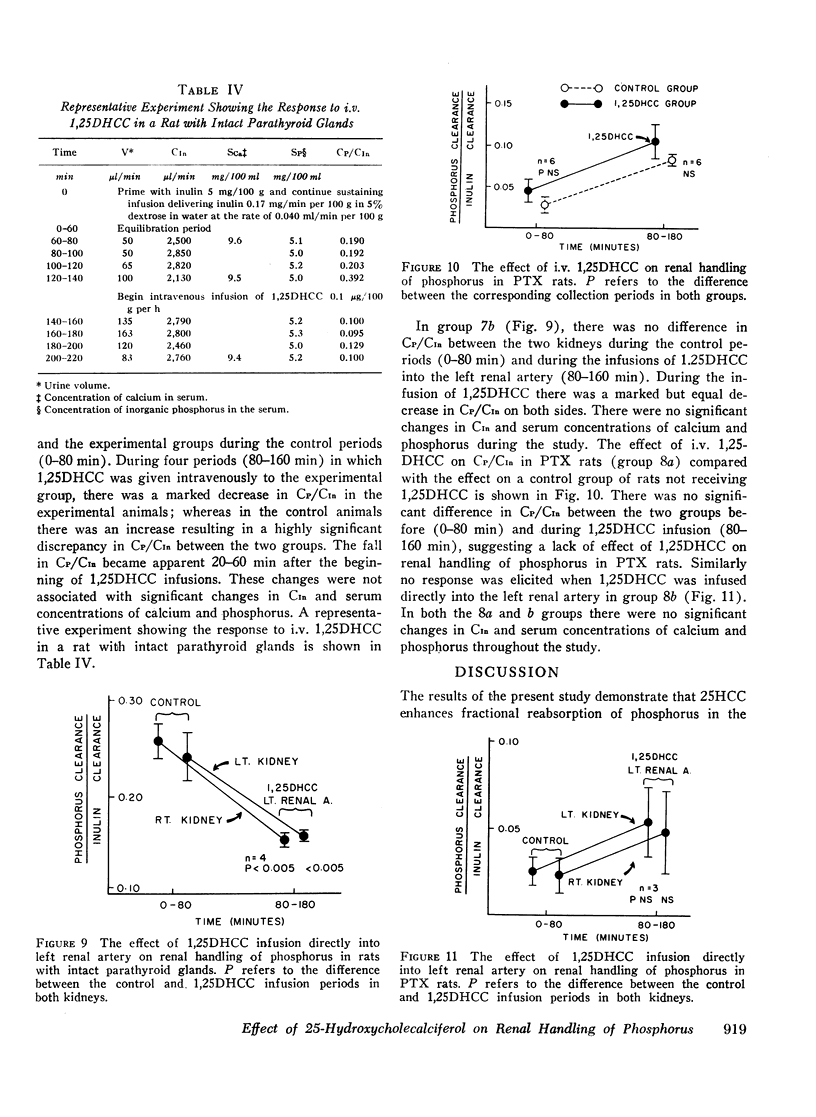
In intact animals, i.v. 25HCC decreased CP/CIn from 0.29±0.04 (mean ±SE) to 0.19±0.04, and i.v. 1,25-DHCC decreased CP/CIn from 0.25±0.04 to 0.15±0.02. The intrarenal infusion of both 25HCC and 1,25DHCC into intact animals failed to produce a unilateral change; however, it decreased CP/CIn bilaterally. i.v. and intrarenal infusions of 25HCC or 1,25DHCC in PTX hypocalcemic and normocalcemic rats, and i.v. infusions of 25HCC in PTX rats receiving either sodium phosphate or normal saline, all failed to produce significant changes in CP/CIn. In contrast, 24HCC given i.v. to PTX animals receiving exogenous PTH was associated with a significant fall in CP/CIn, from 0.34±0.08 to 0.13±0.02. These results indicate that 25HCC enhances tubular reabsorption of phosphorus in rats, only in the presence of either endogenous or exogenous circulating PTH, but not in its absence and thus imply a PTH-dependent mechanism of 25HCC action on the kidney. This effect does not appear to be related to the conversion of 25HCC into 1,25DHCC, since the latter fails to affect tubular reabsorption of phosphorus in PTX rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud C., Rasmussen H., Anast C. Further studies on the interrelationship between parathyroid hormone and vitamin D. J Clin Invest. 1966 Dec;45(12):1955–1964. doi: 10.1172/JCI105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLOR C. H., VAN ALSTINE H. E., KEUTMANN E. H., BASSETT S. H. The fate of intravenously administered calcium; effect on urinary calcium and phosphorus, fecal calcium and calcium-phosphorus balance. J Clin Invest. 1950 Sep;29(9):1167–1176. doi: 10.1172/JCI102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzeville C. Catheterization of renal artery in rats. Proc Soc Exp Biol Med. 1968 Dec;129(3):932–936. doi: 10.3181/00379727-129-33462. [DOI] [PubMed] [Google Scholar]
- Bordier P., Hioco D., Rouquier M., Hepner G. W., Thompson G. R. Effects of intravenous vitamin D on bone and phosphate metabolism in osteomalacia. Calcif Tissue Res. 1969 Aug 11;4(1):78–83. doi: 10.1007/BF02279108. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- CRAWFORD J. D., GRIBETZ D., TALBOT N. B. Mechanism of renal tubular phosphate reabsorption and the influence thereon of vitamin D in completely parathyroidectomized rats. Am J Physiol. 1955 Jan;180(1):156–162. doi: 10.1152/ajplegacy.1954.180.1.156. [DOI] [PubMed] [Google Scholar]
- Chu H. I., Liu S. H., Yu T. F., Hsu H. C., Cheng T. Y., Chao H. C. CALCIUM AND PHOSPHORUS METABOLISM IN OSTEOMALACIA X. FURTHER STUDIES ON VITAMIN D ACTION: EARLY SIGNS OF DEPLETION AND EFFECT OF MINIMAL DOSES. J Clin Invest. 1940 Mar;19(2):349–363. doi: 10.1172/JCI101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG E. EFFECTS OF SERUM CALCIUM LEVEL AND PARATHYROID EXTRACTS ON PHOSPHATE AND CALCIUM EXCRETION IN HYPOPARATHYROID PATIENTS. J Clin Invest. 1965 Jun;44:942–946. doi: 10.1172/JCI105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A. Parathormone as a mediator of inorganic phosphate diuresis during saline infusion in the rat. Pflugers Arch. 1971;325(1):1–13. doi: 10.1007/BF00587487. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Deluca H. F. 1,25-dihydroxycholecalciferol: the metabolite of vitamin D responsible for increased intestinal calcium transport. Arch Biochem Biophys. 1971 Nov;147(1):143–147. doi: 10.1016/0003-9861(71)90320-1. [DOI] [PubMed] [Google Scholar]
- Galli A. Dosage colorimétrique de l'inuline dans le sang et dans les urines. Pathol Biol. 1966 Oct;14(19):991–993. [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux F. H., Scriver C. R., Reade T. M., Goldman H., Roseborough A. Use of phosphate and vitamin D to prevent dwarfism and rickets in X-linked hypophosphatemia. N Engl J Med. 1972 Sep 7;287(10):481–487. doi: 10.1056/NEJM197209072871003. [DOI] [PubMed] [Google Scholar]
- Glorieux F., Scriver C. R. Loss of a parathyroid hormone-sensitive component of phosphate transport in X-linked hypophosphatemia. Science. 1972 Mar 3;175(4025):997–1000. doi: 10.1126/science.175.4025.997. [DOI] [PubMed] [Google Scholar]
- HOWARD J. E., HOPKINS T. R., CONNOR T. B. On certain physiologic responses to intravenous injection of calcium salts into normal hyperparathyroid and hypoparathyroid persons. J Clin Endocrinol Metab. 1953 Jan;13(1):1–19. doi: 10.1210/jcem-13-1-1. [DOI] [PubMed] [Google Scholar]
- Harrison H. E., Harrison H. C. THE RENAL EXCRETION OF INORGANIC PHOSPHATE IN RELATION TO THE ACTION OF VITAMIN D AND PARATHYROID HORMONE. J Clin Invest. 1941 Jan;20(1):47–55. doi: 10.1172/JCI101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972 Jun 9;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Lavender A. R., Pullman T. N. Changes in inorganic phosphate excretion induced by renal arterial infusion of calcium. Am J Physiol. 1963 Nov;205(5):1025–1032. doi: 10.1152/ajplegacy.1963.205.5.1025. [DOI] [PubMed] [Google Scholar]
- Morgan D. B., Paterson C. R., Woods C. G., Pulvertaft C. N., Fourman P. Osteomalacia after gastrectomy. A response to very small doses of vitamin D. Lancet. 1965 Nov 27;2(7422):1089–1091. doi: 10.1016/s0140-6736(65)90061-9. [DOI] [PubMed] [Google Scholar]
- Ney R. L., Kelly G., Bartter F. C. Actions of vitamin D independent of the parathyroid glands. Endocrinology. 1968 Apr;82(4):760–766. doi: 10.1210/endo-82-4-760. [DOI] [PubMed] [Google Scholar]
- Popovtzer M. M., Massry S. G., Villamil M., Kleeman C. R. Renal handling of phosphorus in oliguric and nonoliguric mercury-induced acute renal failure in rats. J Clin Invest. 1971 Nov;50(11):2347–2354. doi: 10.1172/JCI106733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovtzer M. M., Robinette J., Halgrimson C. G., Starzl T. E. Acute effect of prednisolone on renal handling of sodium. Am J Physiol. 1973 Mar;224(3):651–658. doi: 10.1152/ajplegacy.1973.224.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Moranz J., Kurnick W. S. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972 Feb;51(2):373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN H., DELUCA H., ARNAUD C., HAWKER C., VONSTEDINGK M. THE RELATIONSHIP BETWEEN VITAMIN D AND PARATHYROID HORMONE. J Clin Invest. 1963 Dec;42:1940–1946. doi: 10.1172/JCI104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Simmons H. Induction of bone resorption in tissue culture. Prolonged response after brief exposure to parathyroid hormone or 25-hydroxycholecalciferol. Endocrinology. 1972 Mar;90(3):744–751. doi: 10.1210/endo-90-3-744. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. G., Norman A. W., Reddy C. R., Coburn J. W. Biologic effects of 1,25-dihydroxycholecalciferol (a highly active vitamin D metabolite) in acutely uremic rats. J Clin Invest. 1972 May;51(5):1287–1291. doi: 10.1172/JCI106923. [DOI] [PMC free article] [PubMed] [Google Scholar]


