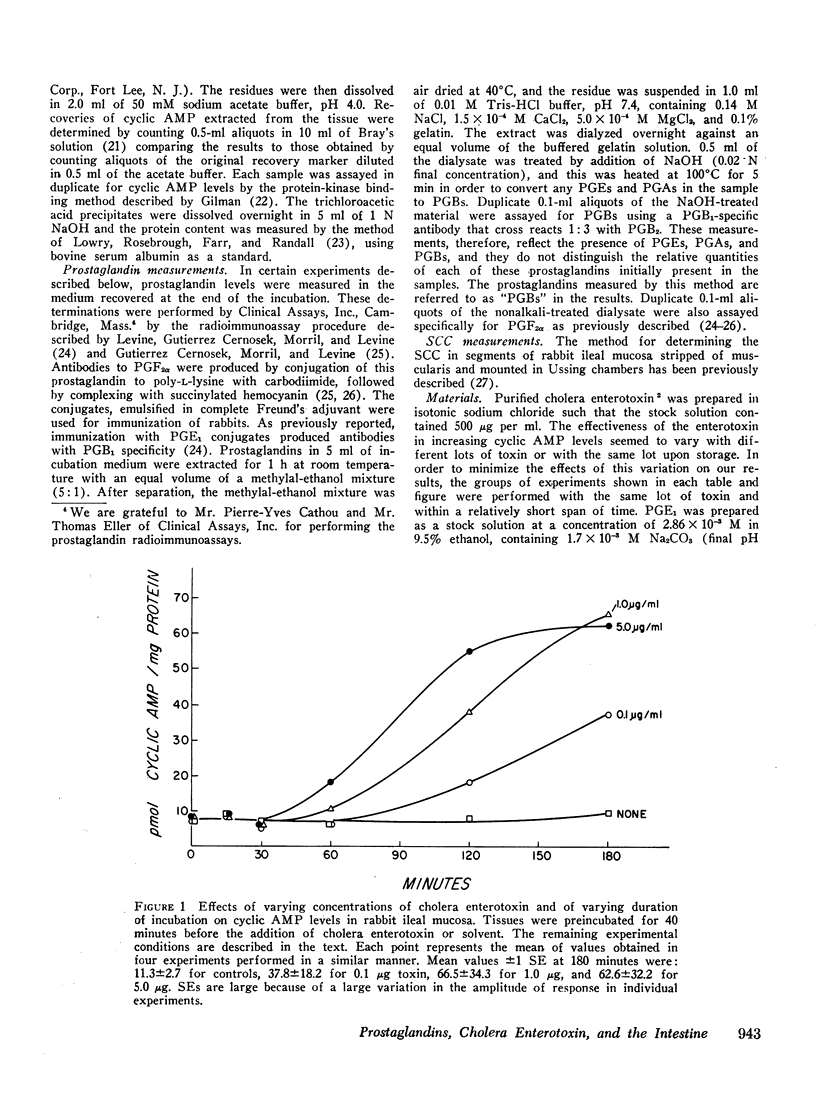
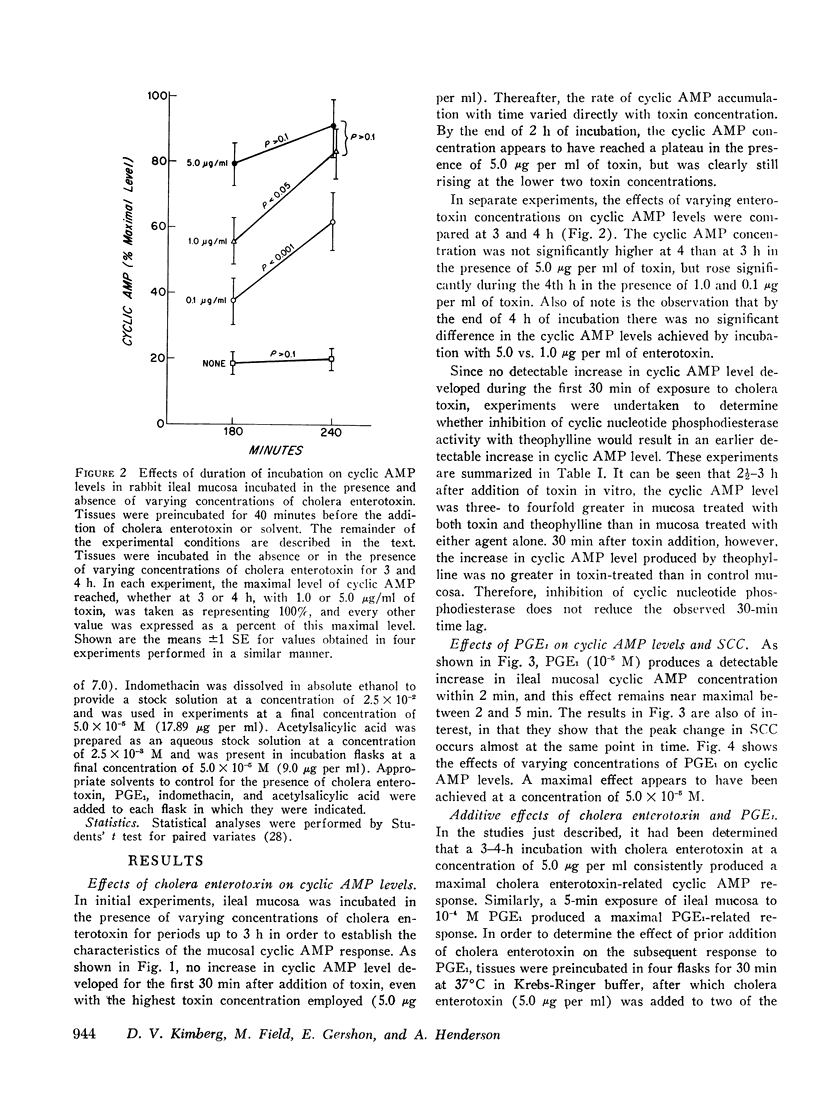
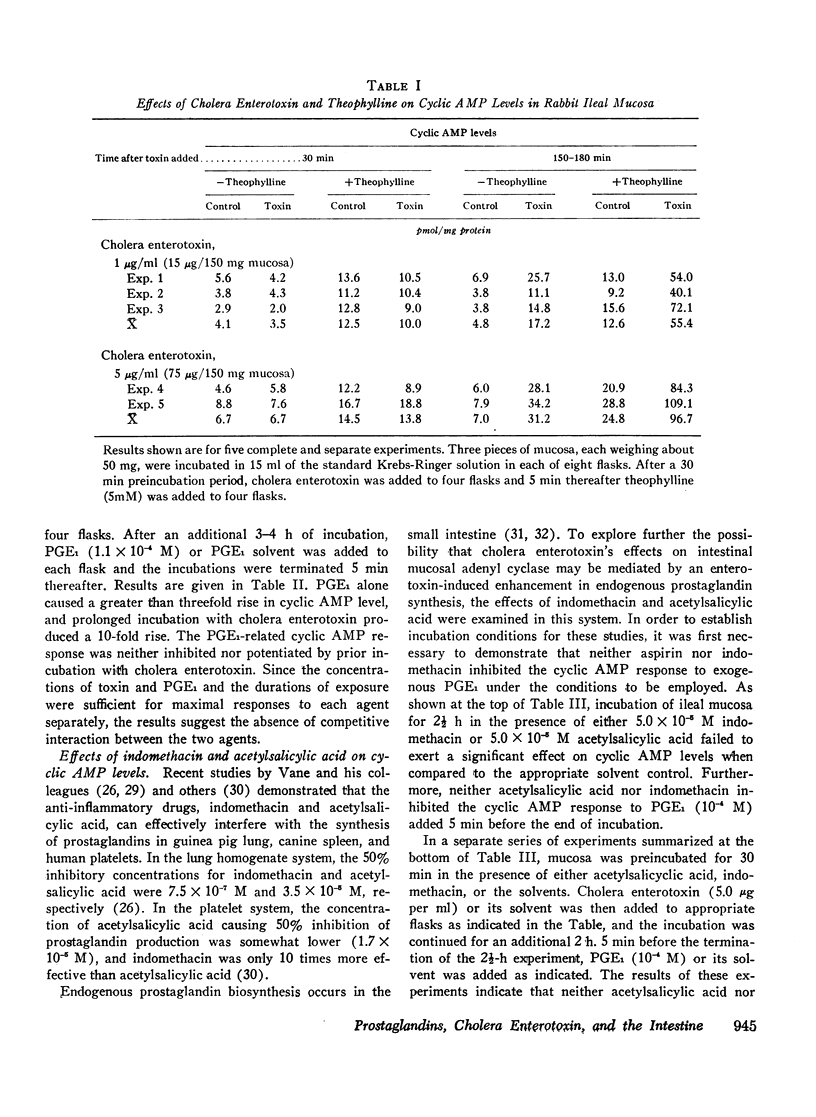
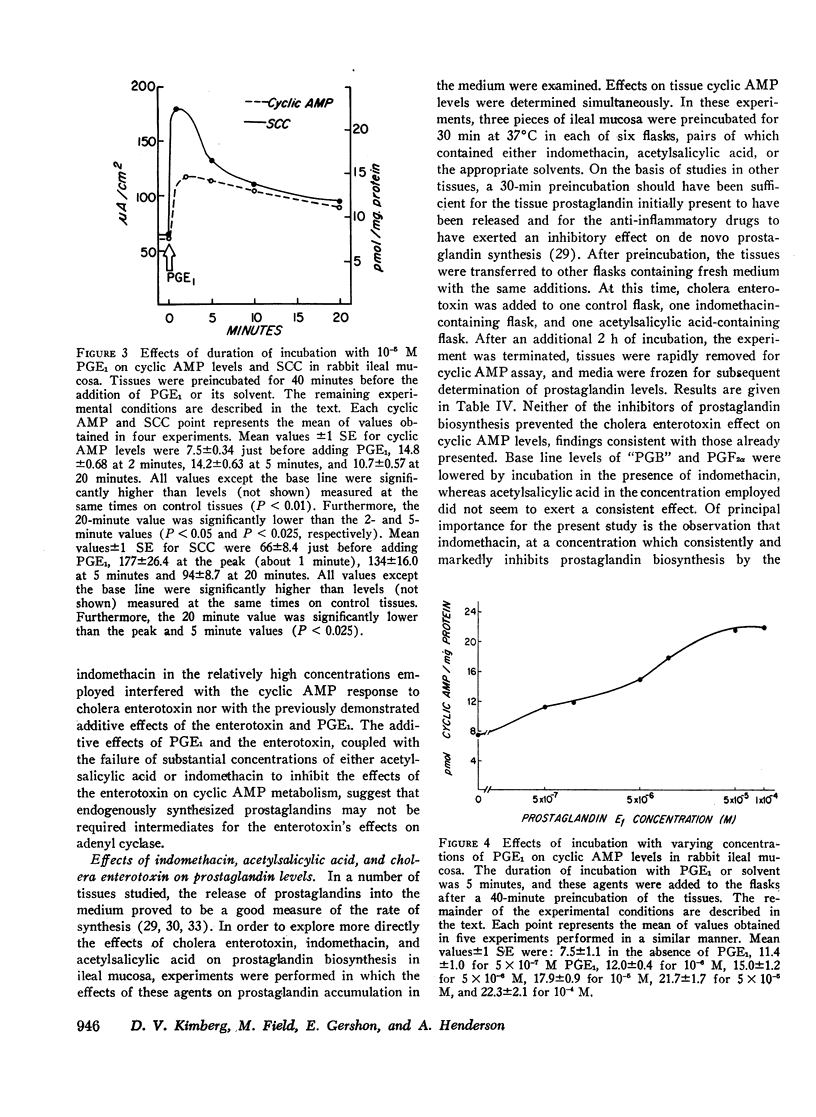
Abstract
Both cholera enterotoxin and certain prostaglandins have been shown to stimulate intestinal fluid secretion in vivo, to cause ion flux changes in vitro similar to those caused by addition of cyclic 3′,5′-adenosine monophosphate (cyclic AMP), and to activate intestinal mucosal adenyl cyclase. It has been suggested that the effects of the enterotoxin on intestinal cyclic AMP metabolism may be indirect, and that locally synthesized prostaglandins may serve as required intermediates for the effects of the enterotoxin in activating intestinal mucosal adenyl cyclase. In order to clarify certain aspects of the mechanisms by which these two agents alter intestinal mucosal cyclic AMP metabolism and ion transport, their effects on cyclic AMP accumulation in rabbit ileal mucosa were examined in vitro. Addition of 5 μg per ml (75 μg per 150 mg mucosa) of purified cholera enterotoxin produced a peak increase in cyclic AMP level in 3 h but there was a time delay of at least 30 min before any effect was observed. Inhibition of cyclic nucleotide phosphodiesterase with theophylline failed to reduce this time delay. In contrast, addition of prostaglandin E1 (PGE1) increased the cyclic AMP level rapidly, a peak effect being observed in 2 min. The time of the peak prostaglandin-induced changes in cyclic AMP level and short-circuit current correlated closely. A maximal increment in cyclic AMP level was achieved with 5 × 10−5 M PGE1. When 10−4 M PGE1 was added to mucosa already maximally stimulated with cholera toxin, the resulting cyclic AMP level was equal to the sum of the levels reached when each agent was added alone. Furthermore, the effects of the enterotoxin on mucosal cyclic AMP levels were not influenced by indomethacin under conditions where mucosal prostaglandins synthesis was inhibited. The results suggest that endogenous prostaglandins do not provide an essential link in the activation of intestinal mucosal adenyl cyclase by cholera enterotoxin. The present study also indicates that the effect of cholera enterotoxin on intestinal mucosal cyclic AMP metabolism involves a definite time delay which is not due to cyclic nucleotide phosphodiesterase activity.
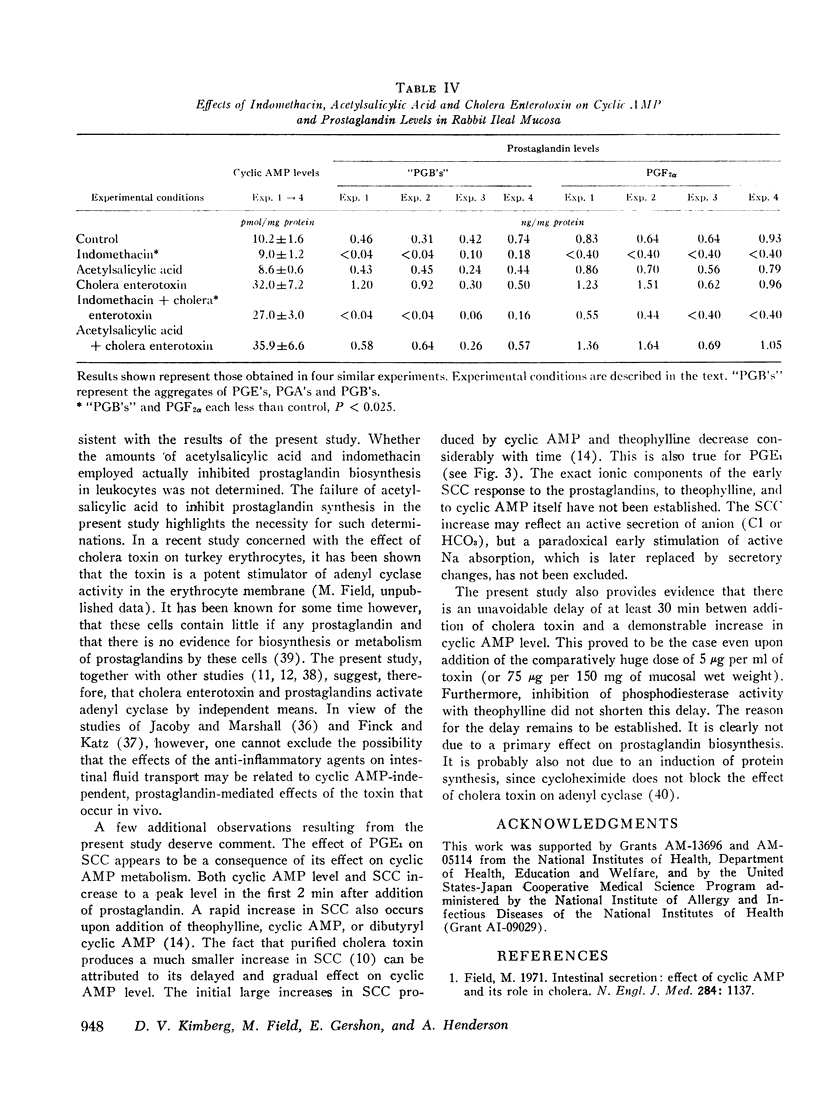
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. Cholera and prostaglandins. Nature. 1971 Jun 25;231(5304):536–536. doi: 10.1038/231536a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. Cholera enterotoxin: failure of anti-inflammatory agents to prevent cyclic AMP accumulation. Nature. 1973 Feb 9;241(5389):399–399. doi: 10.1038/241399a0. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Greenough W. B., 3rd, Sack R. B. The relationship of superior mesenteric artery blood flow to gut electrolyte loss in experimental cholera. J Infect Dis. 1969 Feb;119(2):182–193. doi: 10.1093/infdis/119.2.182. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Jr Cholera enterotoxin--recent investigations yield insights into transport processes. Am J Med. 1971 Jan;50(1):1–7. doi: 10.1016/0002-9343(71)90198-7. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott H. L., Carpenter C. C., Sack R. B., Yardley J. H. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Field M: Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971 May 20;284(20):1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Finck A. D., Katz R. L. Prevention of cholera-induced intestinal secretion in the cat by aspirin. Nature. 1972 Aug 4;238(5362):273–274. doi: 10.1038/238273a0. [DOI] [PubMed] [Google Scholar]
- GANGAROSA E. F., BEISEL W. R., BENYAJATI C., SPRINZ H., PIYARATN P. The nature of the gastrointestinal lesion in asiatic cholera and its relation to pathogenesis: a biopsy study. Am J Trop Med Hyg. 1960 Mar;9:125–135. doi: 10.4269/ajtmh.1960.9.125. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Jacoby H. I., Marshall C. H. Antagonism of cholera enterotoxin by anti-inflammatory agents in the rat. Nature. 1972 Jan 21;235(5334):163–165. doi: 10.1038/235163a0. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Schooley R. T., Henderson A. Effects of cycloheximide on the response of intestinal mucosa to cholera enterotoxin. J Clin Invest. 1973 Jun;52(6):1376–1383. doi: 10.1172/JCI107310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L., Tarnoff J., Cirillo V. J., Ham E. A. Prostaglandin receptor site: evidence for an essential role in the action of luteinizing hormone. Science. 1970 Aug 28;169(3948):883–886. doi: 10.1126/science.169.3948.883. [DOI] [PubMed] [Google Scholar]
- Kunze H. Formation of [I-14C] prostaglandin E2 and two prostaglandin metabolites from [I-14C] arachidonic acid during vascular perfusion of the frog intestine. Biochim Biophys Acta. 1970 Feb 10;202(1):180–183. doi: 10.1016/0005-2760(70)90229-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Matuchansky C., Bernier J. J. Effect of prostaglandin E 1 on glucose, water, and electrolyte absorption in the human jejunum. Gastroenterology. 1973 Jun;64(6):1111–1118. [PubMed] [Google Scholar]
- Ozer A., Sharp G. W. Effect of prostaglandins and their inhibitors on osmotic water flow in the toad bladder. Am J Physiol. 1972 Mar;222(3):674–680. doi: 10.1152/ajplegacy.1972.222.3.674. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Shaw J., Gibson W., Jessup S., Ramwell P. The effect of PGE-1 on cyclic AMP and ion movements in turkey erythrocytes. Ann N Y Acad Sci. 1971 Apr 30;180:241–260. doi: 10.1111/j.1749-6632.1971.tb53195.x. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Vaisrub S. Cholera, prostaglandins, and CAMP. JAMA. 1972 Jan 10;219(2):213–213. doi: 10.1001/jama.219.2.213b. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]


