Abstract
The statistical association between endurance exercise capacity and cardiovascular disease suggests that impaired aerobic metabolism underlies the cardiovascular disease risk in men and women. To explore this connection, we applied divergent artificial selection in rats to develop low-capacity runner (LCR) and high-capacity runner (HCR) rats and found that disease risks segregated strongly with low running capacity. Here, we tested if inborn low aerobic capacity promotes differential sex-related cardiovascular effects. Compared with HCR males (HCR-M), LCR males (LCR-M) were overweight by 34% and had heavier retroperitoneal, epididymal, and omental fat pads; LCR females (LCR-F) were 20% heavier than HCR females (HCR-F), and their retroperitoneal, but not perireproductive or omental, fat pads were heavier as well. Unlike HCR-M, blood pressure was elevated in LCR-M, and this was accompanied by left ventricular (LV) hypertrophy. Like HCR-F, LCR-F exhibited normal blood pressure and LV weight as well as increased spontaneous cage activity compared with males. Despite normal blood pressures, LCR-F exhibited increased myocardial interstitial fibrosis and diastolic dysfunction, as indicated by increased LV stiffness, a decrease in the initial filling rate, and an increase in diastolic relaxation time. Although females exhibited increased arterial stiffness, ejection fraction was normal. Increased interstitial fibrosis and diastolic dysfunction in LCR-F was accompanied by the lowest protein levels of phosphorylated AMP-actived protein kinase [phospho-AMPK (Thr172)] and silent information regulator 1. Thus, the combination of risk factors, including female sex, intrinsic low aerobic capacity, and overweightness, promote myocardial stiffness/fibrosis sufficient to induce diastolic dysfunction in the absence of hypertension and LV hypertrophy.
Keywords: obesity, cardiovascular disease, exercise, activity
in the last several decades, societies have developed the capacity to produce stabile and abundant food supplies and labor-saving conveniences that have resulted in a per capita decrease in essential physical activity. The unfortunate consequence of the decline in physical activity, combined with the propensity to overconsume foods high in fat and simple sugars, is inciting a worldwide epidemic of obesity and the associated increases in cardiovascular morbidity and mortality (5, 16, 40). Furthermore, obesity and diabetes are powerful risk factors for cardiovascular disease in women (37). Obesity is a major contributor to the development of metabolic syndrome, which is generally defined as some combination of comorbidities such as hypertension, insulin resistance, dyslipidemia, and increased visceral adiposity (40). Furthermore, obesity is an independent risk factor for left ventricular (LV) dysfunction and heart failure, independent of other associated comorbidities such as hypertension and coronary heart disease (34). The early myocardial response to hyperlipidemia involves an increased reliance on fatty acids (FAs), which results in increased β-oxidation and mitochondrial accumulation of acyl carnitines that lead to an uncoupling of oxidative phosphorylation (7, 11, 31). Increased β-oxidation has a suppressing effect on myocyte carbohydrate utilization by inhibition of cytosolic glycolysis and mitochondrial pyruvate dehydrogenase. The overreliance on FAs for energy can lead to reduced cardiac efficiency, i.e., work performed per unit O2 consumption. A study (37) of obese inactive women with dyslipidemia has shown that they manifest increased cardiac FA uptake and oxidation before the development of systolic dysfunction. Regular aerobic exercise may be an effective therapeutic option to prevent or reverse metabolic syndrome and the associated heart disease (18).
To elucidate the relationships between aerobic capacity and chronic metabolic disease, Koch and Britton (22, 28, 52) developed two strains of rats with marked differences in intrinsic aerobic endurance exercise capacity. This was accomplished by selective breeding of rats that exhibited either high or low treadmill endurance running capacity. The strains are referred to as high-capacity runners (HCR) or low-capacity runners (LCR). Both strains were developed using rats that were sedentary (caged activity-only condition) to factor out potential confounding influences of daily exercise training. By generation 6, there was a 171% divergence in running capacity, with most of the change in running capacity relative to the founder population occurring in the HCR group (−13% in LCR and +136% in HCR) (28). The divergence in running capacity was accompanied by a divergence in body weight (BW); specifically, LCR male (LCR-M) and LCR female (LCR-F) rats were 16% and 20% heavier than their HCR counterparts (HCR-M and HCR-F, respectively). By generation 11, LCR-F rats were mildly hypertensive and LCR-M rats were insulin resistant (52). LCR rats also exhibited reduced hepatic mitochondrial oxidative capacity and steatosis (45). Moreover, high-fat diets increased the risks for the development of cardiovascular, metabolic, and renal abnormalities in LCR rats (4, 35). Thus, LCR rats exhibit metabolic and cardiovascular phenotypes (i.e., overweight, insulin resistance, elevated blood pressure, and dyslipidemia). Cardiac gene expression profiling indicated the differential expression of numerous genes, especially those related to lipid and glucose metabolism (8). Nonetheless, the functional consequences of differential gene expression in female and male LCR and HCR hearts have not been examined in detail. In this study, we examined differential cardiac structure, metabolic signaling, and function in sedentary female and male 30-wk-old LCR and HCR rats. We hypothesized that low aerobic capacity increases the risk for the development of abnormal cardiac remodeling and diastolic and/or systolic dysfunction. We further posited that there would be a sex-related impact on this differential cardiac functional impact of exercise (22, 23). Here, we report that normotensive LCR-F rats exhibit evidence of increased interstitial fibrosis and diastolic dysfunction.
METHODS
Animal Models and Procedures
The development of rats artificially selected to be either LCR or HCR was originally described by Koch and Britton (28) and Wisloff et al. (52). In the present investigation, male and randomly cycling female HCR and LCR rats (generation 27) were housed under standard temperature and humidity laboratory conditions in which the light and dark cycles were 12 h each. Rats were tested for running capacity at the University of Michigan at 11 wk of age and shipped to the University of Missouri at 16 wk of age. Animals were cared for in accordance with National Institutes of Health guidelines, and all procedures were approved in advance by the Harry S Truman Veterans Memorial Hospital Subcommittee for Animal Safety as well as by the Institutional Animal Care and Use Committee of the University of Missouri.
Telemetric Blood Pressure and Baroreflex Sensitivity Monitoring
Under isoflurane anesthesia (2% isoflurane in a stream of air containing 40% O2), a subset of HCR and LCR rats (26 wk of age) were implanted with an abdominal aorta catheter attached to a radiotransmitter (TA11PA-C40, Data Sciences, St. Paul, MN) as previously described (15). After 3 wk of recovery, rats were monitored in 300-s bins every 15 min for three 12-h light and three 12-h dark cycles (sampling rate: 1,000 Hz). The parameters evaluated included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), and spontaneous cage activity [in counts of lateral movement per minute (cpm)] (3, 24). Telemetry data from male and female HCR and LCR rats were analyzed post hoc for spontaneous baroreflex sensitivity using the sequence method (3) with the aid of freely available HemoLab software (http://www.haraldstauss.com/HemoLab/HemoLab.php) as previously described (24). Analysis was performed on bins of 300 s collected every 30 min for three 12-h light and three 12-h dark cycles. Baroreflex sequences were identified as ramps of 4+ consecutive beats in which both SBP and the interpulse interval increased or decreased simultaneously, and spontaneous baroreflex sensitivity was calculated via linear regression (R for inclusion: 0.8) as the slope (in ms/mmHg) of that relationship.
Cardiac Catheterization and Cardiac Function Testing
Cardiac catheterization to examine LV diastolic and systolic function was performed on 30-wk-old rats under isoflurane anesthesia (as described above) as previously described using an Advantage PV System (model FY897B, Scisense) (15). The steps involved in the setup and use of the Advantage system, i.e., catheter calibration and measures of myocardial conductivity and permittivity, have been previously described (30). During data acquisition, the small animal ventilator (SAR 1000, Charles Ward Enterprises, Ardmore, PA) was turned off for 5–6 s to eliminate lung motion artifacts (36). Despite the invasive nature of the technique, direct catheterization and simultaneous measurements of both pressure and volume are the most widely used approach to interrogate cardiac function in rodents (12, 36). Noninvasive techniques, such as echo or MRI, are limited to analyses performed under steady-state conditions and by reliance on motion parameters that can be affected by preload or afterload, ventricular geometry, or HR. Thus, pressure-volume analysis is considered to be the gold standard for the measurement of systolic and diastolic function largely due to the capability of this technique to generate indexes of cardiac function that are load independent. In addition, this technique enables the quantitative examination of chamber energetics and cardiovascular interactions (12).
In Vivo Cine MRI
Noninvasive cardiac MRI scans have superior spatial and temporal resolutions that permit visualization of the entire heart, enabling an accurate estimation of cardiac dimensions and volumes over time (13). Furthermore, generation of blood velocity profiles at any arbitrary point within the ventricle cavity or wall enable the assessment of several systolic and diastolic parameters without certain limitations of Doppler echocardiography related to beam optimization. Imaging was performed on animals under isoflurane anesthesia (as described above) within 72 h of the end of the treatment period using a Varian 7-T horizontal-bore MRI (Varian, Palo Alto, CA) equipped with a 60-mm birdcage radiofrequency coil as previously described (20, 54). Briefly, ECG and respiratory monitoring and gating were acquired with a small animal monitoring system (SA Instruments, Stony Brook, NY) with 1-mm slice thickness and 65 × 45- and 45 × 45-mm2 fields of view for the LV in long- and short-axis images, respectively. Septal wall thickness measurements were determined on the midventricular axial image immediately after the R wave and with an averaging of five measurements equally spaced along the septum. LV functional parameters were determined using a series of cine images of the LV in the long-axis view acquired at 16 equally spaced time points throughout the entire cardiac cycle with a frame rate of 8–12 ms/frame. The first derivatives of the LV volume against time curve were calculated to extract the diastolic filling rates and relaxation time. The diastolic initial filling rate was defined as the slope of the first four time points of the early diastolic curve. The diastolic peak filling rate was defined as the maximum derivative of the LV volume curve. The diastolic relaxation time was defined as the time duration from the end of the systolic phase to the peak filling phase (54).
Histomorphometric and Immunohistochemical Analysis
Thirty-week-old rats were weighed and euthanized via exsanguination under isoflurane anesthesia (as described above). At autopsy, the LV and septum were dissected, and the wet weight-to-BW ratio (LV + septum/BW) was determined. A portion of the LV was immersion fixed in 3% paraformaldehyde, dehydrated in ethanol, paraffin embedded, and then sectioned in the transverse plane at 5 μm. Samples from five rats from each of the four groups were analyzed for interstitial fibrosis and ultrastructure as previously described (20). As an additional assessment of overweight/obesity, the retroperitoneal, omental, and perireproductive (epididymal for males and ovarian/uterine for females) fat pads were dissected free of other tissue and weighed immediately.
Light microscopic analysis for myocardial interstitial fibrosis.
After rehydration in ethanol and HEPES buffer, paraffin sections were stained with Verhoeff van Gieson stain, which stains collagen fibers pink, to evaluate interstitial fibrosis. The amount of collagen was calculated as a percentage of the total area within five representative region of interest rectangles per animal with the aid of the thresholding function in MetaVue software.
Immunohistochemistry.
LV tissue was fixed, embedded in paraffin, and immunostained with an antibody to silent information regulator (sirtuin)1 (Sirt1; 1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (51). Immunostained slides were examined using a laser confocal scanning microscope (Bio-Rad, Cambridge, MA), images were captured using Laser-sharp software (Bio-Rad), and signal intensities were measured by MetaVue (Boyce Scientific, Gray Summit, MO).
Ultrastructure analysis with transmission electron microscopy.
Details of LV tissue preparation, sectioning, staining, and viewing are as previously described (50). A JOEL 1200-EX transmission electron microscope was used to review three fields randomly chosen per rat to obtain three ×60,000 images/LV.
Immunoblot Analysis
Western blot analyses were performed for the assessment of carnitine palmitoyltransferase 1 (CPT1; Santa Cruz Biotechnology), oxidative phosphorylation (OXPHOS) complexes I–V of the electron transport chain (MitoProfile Total OXPHOS Rodent WB Antibody Cocktail, MitoSciences, Eugene, OR), AMP-activated protein kinase-α (AMPK; Cell Signaling Technology, Beverly, MA), Thr172-phosphorylated AMPK-α [p-AMPK(T172); Cell Signaling Technology,], and β-actin (Santa Cruz Biotechnology). Briefly, samples were homogenized in homogenization buffer containing 250 mM sucrose, 50 mM HEPES, 0.5 mM EDTA, one protease inhibitor cocktail tablet, 200 mM sodium orthovanadate, and 50 mM sodium pyrophosphate. Homogenates were centrifuged at 2,500 g for 10 min. Insoluble pellets were discarded, and supernatants were again ultracentrifuged at 19,000 g for 60 min. The resulting pellets were collected as enriched mitochondrial fractions, and the supernatants were again ultracentrifuged at 33,000 g for 90 min. The final supernatant was collected as the cytosolic fraction. Okadaic acid (Acros Organics, Morris Plains, NJ) was added to each mitochondrial and cystosolic sample at a final concentration of 0.1 μM. Protein concentrations were determined using a BCA protein assay kit. Cytosolic [for AMPK, p-AMPK(T172), and β-actin] fractions and enriched mitochondrial (for OXPHOS and CPT1) fractions (30 μg/lane) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). AMPK, p-AMPK(T172), CPT1, and β-actin blots were incubated 1 h with 5% BSA in Tris-buffered saline with Tween 20 (TBST) at room temperature and then for 1 h at room temperature with primary antibodies (1:1,000, 1:1,000, 1:500, and 1:2,000 dilution, respectively) in 5% BSA, rinsed five times with TBST, and incubated with horseradish peroxidase-conjugated secondary antibodies (1:30,000 dilution of each antibody) in 5% BSA for 1 h at room temperature. The OXPHOS blot was blocked in 5% milk overnight at 4°C, incubated in primary antibody (1:1,000 dilution) in 5% BSA overnight at 4°C, rinsed five times in TBST, and incubated with horseradish peroxidase-conjugated secondary antibody (1:500 dilution) in 5% milk for 1 h at room temperature. Binding of the antibodies was detected by chemiluminescence, and images were recorded using a Bio-Rad ChemiDoc XRS image-analysis system. Quantitation of protein band density, normalized to the density of total protein via amido black stain for enriched mitochondrial fractions or β-actin for cytosolic fractions, was performed using Image Lab software (Bio-Rad). Data are reported as normalized protein band densities in arbitrary units.
Quantitative Analysis of Mitochondrial Enzyme levels and Activity
Citrate synthase activity.
Citrate synthase is a Krebs cycle enzyme used to assess the aerobic capacity of mitochondria (41). LVs from animals were removed and homogenized as described above to obtain cytosolic and enriched mitochondrial fractions. Mitochondrial fractions were resuspended in sucrose homogenization buffer composed of sucrose, HEPES, EDTA, a complete EDTA-free protease inhibitor cocktail tablet (Roche), sodium pyrophosphate, and sodium orthovanadate. Protein content was determined with a BCA protein assay kit (Thermo Scientific). Citrate synthase activity was determined as previously described (41). Briefly, 10 μg of mitochondrial protein were incubated with buffer containing 66.5 mM Tris (pH 8.3), 0.5 mM oxaloacetate, 0.43 mM acetyl-CoA, and 95 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) at 37°C. Spectrophotometric detection of reduced DTNB at a wavelength of 412 nm was measured every 20 s for 10 min. The amount of DTNB converted per minute was determined with the molar extinction coefficient for DTNB. The maximum slope was calculated and used as an indication of citrate synthase activity.
β-Hydroxyacyl-CoA dehydrogenase activity.
β-Hydroxyacyl-CoA dehydrogenase (β-HAD) is an enzyme involved in mitochondrial FA β-oxidation. β-HAD activity was measured as previously described with modifications (1). Briefly, 10 μg of LV mitochondrial protein were incubated in assay buffer containing 0.1 M triethanolamine-HCl, 5 mM EDTA, and 0.45 mM NADH (pH 7.0) at 37°C. After an initial 2-min absorbance reading at 340 nm, the reaction was initiated by adding 0.1 mM acetoacetyl-CoA, and the rates of disappearance of NADH were measured by the changes in absorbance at 340 nm every 10 s for 10 min. The maximum slope was calculated and used as the indication of β-HAD activity. Enzyme activity was expressed as nanomoles per milligram of protein per minute.
Statistical Analysis
Two-way ANOVA and post hoc t-tests (Holm-Sidak) were performed to examine differences in outcomes between male and female LCR and HCR groups. ANOVA main effects included strain (HCR vs. LCR), sex (male vs. female), and the interaction between strain and sex. All differences were considered significant when P < 0.05 (SigmaPlot 12.0, Systat Software). Separate t-tests were performed to test for differences in fat pad weights in cases where there was a significant interaction effect in two-way ANOVA. All data are reported as means ± SE. To examine whether the level of myocardial Sirt1 protein content relates to relative level of interstitial myocardial fibrosis, we performed linear regression analysis.
RESULTS
Baseline Parameters
LCR-M and LCR-F were 29% and 22% heavier than their HCR counterparts, respectively, and tibia lengths (TLs) were 4.95 and 5.89% longer in LCR-M and LCR-F (Table 1). Males had heavier retroperitoneal, peritoneal, and omental fat pad weights than females, and LCR rats had heavier fat pad weights than HCR rats (main effects comparisons). Surprisingly, LCR-F did not have significantly heavier retroperitoneal and perireproductive fat pad weights compared with HCR-F by post hoc analysis. Separate t-tests comparing retroperitoneal and perireproductive fat pad weights between HCR-F and LCR-F indicated that LCR-F had heavier fat pads, 270% heavier in the case of retroperitoneal fat pads (P < 0.001) and 62% heavier (P < 0.05) in the case of perireproductive fat pads. We further tested whether differences were still evident after fat pad weights were normalized to TL. LCR-M had significantly heavier retroperitoneal, perireproductive, and omental fat pads. We observed significantly greater normalized retroperitoneal fat pad weight in LCR-F compared with HCR-F (P < 0.001) by a separate t-test. On the other hand, perireproductive and omental fat pads normalized to TL did not differ between HCR-F and LCR-F. The heavier BW and uniformly enlarged fat pads in males suggest that LCR-M were obese compared with HCR-M. On the other hand, the heavier BW and adjusted retroperitoneal fat weights of LCR-F supports that LCR-F were overweight compared with HCR-F.
Table 1.
Age, body weight, and fat pad weights in 30-wk-old HCR-M, LCR-M, HCR-F, and LCR-F rats
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 18 | 21 | 18 | 18 | |
| Age at death, wk | |||||
| Strain | 0.825 | 30.1 ± 0.1 | 30.0 ± 0.1 | 30.1 ± 0.1 | 30.1 ± 0.1 |
| Sex | 0.356 | ||||
| Interaction | 0.915 | ||||
| Body weight, g | |||||
| Strain | 0.001 | 361 ± 19c | 468 ± 15a,d | 202 ± 4 | 247 ± 4b |
| Sex | 0.001 | ||||
| Interaction | 0.023 | ||||
| TL, mm | |||||
| Strain | 0.001 | 40.4 ± 0.3c | 42.4 ± 0.3a,d | 35.6 ± 0.5 | 37.7 ± 0.3b |
| Sex | 0.001 | ||||
| Interaction | 0.956 | ||||
| Retroperitoneal fat, mg | |||||
| Strain | 0.001 | 4,222 ± 451c | 11,136 ± 1,160a,d | 704 ± 76 | 1,918 ± 245e |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| Retroperitoneal fat/TL, mg | |||||
| Strain | 0.001 | 109 ± 13c | 267 ± 27a,d | 20 ± 2 | 51 ± 7e |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| Perireproductive fat, mg | |||||
| Strain | 0.001 | 3,748 ± 402c | 9,388 ± 936a,d | 477 ± 91 | 771 ± 110e |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| Perireproductive fat/TL, mg | |||||
| Strain | 0.001 | 92 ± 10c | 226 ± 22a,d | 14 ± 3 | 21 ± 3 |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| Omental fat, mg | |||||
| Strain | 0.001 | 833 ± 101c | 1,504 ± 164a,d | 465 ± 100 | 512 ± 71 |
| Sex | 0.004 | ||||
| Interaction | 0.012 | ||||
| Omental fat/TL, mg | |||||
| Strain | 0.001 | 21 ± 3 | 36 ± 4a,d | 13 ± 3 | 14 ± 2 |
| Sex | 0.018 | ||||
| Interaction | 0.022 |
Values are means ± SE. HCR-M, high-capacity runner (HCR) male; LCR-M, low-capacity runner (LCR) male; HCR-F, HCR female; LCR-F, LCR female; TL, tibia length (at autopsy).
P < 0.05, HCR-M vs. LCR-M;
P < 0.05, HCR-F vs. LCR-F;
P < 0.05, HCR-M vs. HCR-F;
P < 0.05, LCR-M vs. LCR-F;
P < 0.05, HCR-F vs. LCR-F (by separate t-test).
Telemetry Measures
SBP, DBP, MAP, HR, and activity of 30-wk-old LCR and HCR rats are shown in Table 2. HCR-M, HCR-F, and LCR-F had similar blood pressures during both light and dark cycles. SBP, DBP, and MAP were elevated during the light and dark cycles of LCR-M compared with HCR-M. For the combined 24-h period, MAP for LCR-M was elevated by 9.2% compared with HCR-M (106.1 ± 1.7 vs. 97.2 ± 2.8 mmHg, P < 0.05). HRs were elevated in HCR-F and LCR-F during both light and dark cycles compared with their male counterparts, although there were no differences in HRs between HCR-F and LCR-F. Spontaneous cage activity during the dark (active) period was approximately double that of activity during the light (rest) period for all rats. Activity during the dark cycle was elevated by 151% and 179% in HCR-F and LCR-F, respectively, compared with their male counterparts.
Table 2.
Telemetry data collected remotely in freely moving, conscious 30-wk-old HCR-M, LCR-M, HCR-F, and LCR-F rats monitored in 300-s bins every 15 min for three 12-h light and three 12-h dark cycles
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 8 | 9 | 8 | 8 | |
| Sysolic blood pressure, mmHg | |||||
| Light | |||||
| Strain | 0.059 | 117 ± 4 | 126 ± 2 | 118 ± 3 | 120 ± 2 |
| Sex | 0.421 | ||||
| Interaction | 0.202 | ||||
| Dark | |||||
| Strain | 0.023 | 122 ± 4 | 131 ± 2a | 122 ± 3 | 125 ± 2 |
| Sex | 0.294 | ||||
| Interaction | 0.220 | ||||
| Diastolic blood pressure, mmHg | |||||
| Light | |||||
| Strain | 0.017 | 78 ± 2 | 87 ± 1a | 80 ± 2 | 83 ± 3 |
| Sex | 0.362 | ||||
| Interaction | 0.268 | ||||
| Dark | |||||
| Strain | 0.025 | 83 ± 3 | 90 ± 1a | 83 ± 2 | 87 ± 3 |
| Sex | 0.463 | ||||
| Interaction | 0.458 | ||||
| Mean arterial pressure, mmHg | |||||
| Light | |||||
| Strain | 0.044 | 96 ± 3 | 104 ± 2a | 98 ± 2 | 100 ± 2 |
| Sex | 0.56 | ||||
| Interaction | 0.187 | ||||
| Dark | |||||
| Strain | 0.029 | 100 ± 3 | 108 ± 1a | 101 ± 2 | 104 ± 2 |
| Sex | 0.481 | ||||
| Interaction | 0.286 | ||||
| Heart rate, beats/min | |||||
| Light | |||||
| Strain | 0.108 | 297 ± 8c | 312 ± 8d | 335 ± 8 | 347 ± 6 |
| Sex | 0.001 | ||||
| Interaction | 0.874 | ||||
| Dark | |||||
| Strain | 0.495 | 341 ± 9c | 344 ± 8d | 371 ± 12 | 380 ± 6 |
| Sex | 0.001 | ||||
| Interaction | 0.706 | ||||
| Activity, counts/min | |||||
| Light | |||||
| Strain | 0.066 | 1.34 ± 0.10c | 0.8 ± 0.12d | 1.94 ± 0.20 | 1.74 ± 0.27 |
| Sex | 0.001 | ||||
| Interaction | 0.386 | ||||
| Dark | |||||
| Strain | 0.051 | 2.90 ± 0.3c | 2.0 ± 0.3d | 4.39 ± 0.5 | 3.59 ± 0.5 |
| Sex | 0.001 | ||||
| Interaction | 0.945 | ||||
Values are means ± SE. Comparisons are across groups.
P < 0.05, HCR-M vs. LCR-M;
P < 0.05, HCR-M vs. HCR-F;
P < 0.05, LCR-M vs. LCR-F.
Spontaneous Baroreflex Sensitivity (Sequence Method)
Although more baroreflex sequences were identified in females than males during the dark cycle, LCR-F exhibited fewer baroreflex signals than HCR-F (Table 3). No differences in overall baroreflex sensitivity (gain of all identified baroreflex sequences) were found during either the light or dark cycle. LCR rats displayed diminished reflex bradycardia (up spontaneous baroreflex sensitivity sequences − SBP and interpulse interval increase) compared with HCR rats during the dark, but not light, cycle. No differences in reflex tachycardia (down spontaneous baroreflex sensitivity sequences − SBP and interpulse interval decrease) were found during either the light or dark cycle (Table 3).
Table 3.
Baroreflex gain during light and dark cycles in HCR-M, LCR-M, HCR-F, and LCR-F rats
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 8 | 9 | 8 | 8 | |
| Number of sequences | |||||
| Light | |||||
| Strain | 0.923 | 18.8 ± 2.56 | 17.3 ± 2.82 | 27.1 ± 4.83 | 25.2 ± 3.61 |
| Sex | 0.057 | ||||
| Interaction | 0.868 | ||||
| Dark | |||||
| Strain | 0.183 | 17.8 ± 1.95c | 15.9 ± 1.66 | 30.6 ± 3.53 | 21.7 ± 2.95b |
| Sex | 0.004 | ||||
| Interaction | 0.419 | ||||
| Gain all, ms/mmHg | |||||
| Light | |||||
| Strain | 0.382 | 2.43 ± 0.158 | 2.23 ± 0.247 | 2.26 ± 0.277 | 2.06 ± 0.306 |
| Sex | 0.737 | ||||
| Interaction | 0.882 | ||||
| Dark | |||||
| Strain | 0.133 | 2.32 ± 0.199 | 1.92 ± 0.175 | 2.06 ± 0.231 | 1.75 ± 0.278 |
| Sex | 0.653 | ||||
| Interaction | 0.898 | ||||
| Up sequences, ms/mmHg | |||||
| Light | |||||
| Strain | 0.146 | 2.33 ± 0.163 | 2.10 ± 0.170 | 2.42 ± 0.254 | 1.93 ± 0.250 |
| Sex | 0.986 | ||||
| Interaction | 0.722 | ||||
| Dark | |||||
| Strain | 0.035 | 2.36 ± 0.163 | 1.91 ± 0.129 | 2.16 ± 0.206 | 1.72 ± 0.254 |
| Sex | 0.610 | ||||
| Interaction | 0.841 | ||||
| Down sequences, ms/mmHg | |||||
| Light | |||||
| Strain | 0.606 | 2.46 ± 0.167 | 2.29 ± 0.310 | 2.10 ± 0.314 | 2.14 ± 0.365 |
| Sex | 0.668 | ||||
| Interaction | 0.834 | ||||
| Dark | |||||
| Strain | 0.272 | 2.22 ± 0.234 | 1.89 ± 0.215 | 1.98 ± 0.247 | 1.74 ± 0.302 |
| Sex | 0.762 | ||||
| Interaction | 0.930 | ||||
Values are means ± SE. Parameters shown include the number of sequences identified, gain of all identified sequences (gain all), gain of reflex bradycardia (systolic blood pressure and interpulse interval increase; up sequences), and reflex tachycardia (systolic blood pressure and interpulse interval decrease; down sequences). Comparisons are across groups.
P < 0.05, HCR-F vs. LCR-F;
P < 0.05, HCR-M vs. HCR-F.
Cardiac Function
Pressure-volume analysis.
Pressure-volume analysis performed during steady state indicated no differences in end-diastolic or systolic pressures and volumes, stroke volume, stroke work, ejection fraction, cardiac output, maximum dP/dt (dP/dtmax), or minimum dP/dt (dP/dtmin) (Table 4). The time constant of isovolumic relaxation did not differ among the groups, suggesting no impairments in (Ca2+ reuptake) active properties of relaxation. There were significant strain and sex effects for the cardiac index (CI), a measure of cardiac output normalized to BW, indicating a lower CI in the LCR strain and a higher CI in females. CI was significantly lower in LCR-F compared with HCR-F. Also, there were significant strain and sex effects for the total peripheral resistance (TPR) index, as determined by the following formula: TPR index = MAP/CI, indicating higher TPR in the LCR strain compared with the HCR strain and lower TPR in females compared with males. The TPR index was increased in LCR-M and LCR-F compared with their HCR counterparts, suggesting that the LV of LCR rats, regardless of sex, is subjected to increased systemic vascular resistance. There was a significant sex effect with regard to effective arterial elastance (Ea), indicating that female rats have a net increase in arterial stiffness compared with males; however, no differences in Ea between LCR-F and HCR-F were detected. There was a nearly significant effect of sex on normalized maximum power (P = 0.057), which suggests enhanced systolic function in female rats in response to increased systemic resistance and Ea. Note that normalized maximum power was defined as the maximum instantaneous product of LV pressure and the rate of change of LV volume divided by end-diastolic volume (EDV) squared (26) and that this load-independent index is generated during steady-state pressure-volume loop acquisition. There was a significant sex effect with regard to pressure-volume area (PVA), an index of total mechanical energy generated during ventricular contraction. Specifically, PVA normalized to LV mass indicated that the LV of female rats generated higher mass-adjusted mechanical energy during ventricular contraction than males. Because PVA is highly related to LV O2 consumption per heart beat (42, 43), this analysis indicates that the LV of female rats used more O2 per heartbeat than male rats. Interestingly, PVA/mg was highest in HCR-F, lowest in males of both strains, and intermediate in LCR-F. Nonetheless, an index of myocardial efficiency (efficiency = stroke work/PVA) suggests that there were no differences among the strains and sexes in the relative amount of mechanical energy expended to perform the work required to eject the stroke volume. Thus, at steady state, the increases in measures of peripheral resistance (TPR index) and Ea in both strains of female rats were compensated by increased LV systolic stiffness (elastance), which enabled females to maintain normal ejection fraction. Although the LV of 30-wk-old HCR-F and LCR-F generated more mass-adjusted total mechanical energy (PVA/unit mass), cardiac efficiency (efficiency = work/O2 consumption) was normal.
Table 4.
Steady-state hemodynamic parameters in HCR-M, LCR-M, HCR-F, and LCR-F rats obtained by pressure-volume loop analysis
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 10 | 9 | 8 | 12 | |
| End-systolic pressure, mmHg | |||||
| Strain | 0.175 | 99 ± 8 | 113 ± 8 | 114 ± 5 | 118 ± 5 |
| Sex | 0.145 | ||||
| Interaction | 0.458 | ||||
| End-diastolic pressure, mmHg | |||||
| Strain | 0.463 | 11 ± 2 | 13 ± 2 | 11 ± 1 | 12 ± 2 |
| Sex | 0.853 | ||||
| Interaction | 0.968 | ||||
| End-systolic volume, μl | |||||
| Strain | 0.883 | 163 ± 18 | 151 ± 12 | 124 ± 10 | 140 ± 15 |
| Sex | 0.092 | ||||
| Interaction | 0.346 | ||||
| EDV, μl | |||||
| Strain | 0.965 | 395 ± 39 | 401 ± 30 | 329 ± 16 | 326 ± 23 |
| Sex | 0.015 | ||||
| Interaction | 0.884 | ||||
| Heart rate, beats/min | |||||
| Strain | 0.541 | 301 ± 15 | 297 ± 20 | 322 ± 10 | 308 ± 10 |
| Sex | 0.302 | ||||
| Interaction | 0.745 | ||||
| Stroke volume, μl | |||||
| Strain | 0.961 | 233 ± 25 | 249 ± 21 | 205 ± 14 | 186 ± 15 |
| Sex | 0.020 | ||||
| Interaction | 0.351 | ||||
| Ejection fraction, % | |||||
| Strain | 0.884 | 58 ± 2 | 62 ± 2 | 62 ± 2 | 58 ± 3 |
| Sex | 0.901 | ||||
| Interaction | 0.080 | ||||
| SW, mmHg/μl | |||||
| Strain | 0.777 | 21.0 ± 2.5 | 23.7 ± 2.7 | 19.6 ± 1.1 | 18.1 ± 1.8 |
| Sex | 0.100 | ||||
| Interaction | 0.306 | ||||
| Cardiac output, ml/min | |||||
| Strain | 0.941 | 69 ± 8 | 76 ± 10 | 66 ± 4 | 58 ± 5 |
| Sex | 0.111 | ||||
| Interaction | 0.268 | ||||
| Cardiac index, ml·min−1·g−1 | |||||
| Strain | 0.010 | 0.20 ± 0.02c | 0.17 ± 0.02d | 0.32 ± 0.02 | 0.23 ± 0.02b |
| Sex | 0.001 | ||||
| Interaction | 0.184 | ||||
| Total peripheral resistance index, mmHg·ml−1·min−1·kg−1 | |||||
| Strain | 0.001 | 5.77 ± 0.36d | 8.67 ± 1.26a,d | 3.18 ± 0.26 | 5.29 ± 0.53b |
| Sex | 0.001 | ||||
| Interaction | 0.566 | ||||
| Ea, mmHg/μl | |||||
| Strain | 0.402 | 0.47 ± 0.07 | 0.48 ± 0.05d | 0.58 ± 0.05 | 0.67 ± 0.05 |
| Sex | 0.008 | ||||
| Interaction | 0.443 | ||||
| Arterial-ventricular coupling (Ea/Ees) | |||||
| Strain | 0.803 | 0.32 ± 0.07 | 0.32 ± 0.09 | 0.46 ± 0.12 | 0.42 ± 0.06 |
| Sex | 0.188 | ||||
| Interaction | 0.813 | ||||
| Maximum dP/dt, mmHg/s | |||||
| Strain | 0.477 | 5,440 ± 366 | 6,138 ± 480 | 5,766 ± 491 | 5,734 ± 480 |
| Sex | 0.933 | ||||
| Interaction | 0.435 | ||||
| Minimum dP/dt, mmHg/s | |||||
| Strain | 0.504 | −4,398 ± 398 | −5,057 ± 593 | −5,491 ± 346 | −5,410 ± 378 |
| Sex | 0.099 | ||||
| Interaction | 0.393 | ||||
| Normalized maximum power (SW/PVA) | |||||
| Strain | 0.773 | 3.31 ± 0.68 | 3.36 ± 0.52 | 4.79 ± 0.90 | 5.23 ± 1.03 |
| Sex | 0.057 | ||||
| Interaction | 0.822 | ||||
| Efficiency (SW/PVA) | |||||
| Strain | 0.762 | 0.77 ± 0.05 | 0.73 ± 0.06 | 0.69 ± 0.04 | 0.77 ± 0.07 |
| Sex | 0.737 | ||||
| Interaction | 0.291 | ||||
| PVA/mg, mmHg·μl−1·mg (LV + S)−1 | |||||
| Strain | 0.365 | 42 ± 5c | 42 ± 7 | 60 ± 5 | 49 ± 6 |
| Sex | 0.039 | ||||
| Interaction | 0.359 |
Values are means ± SE. EDV, end-diastolic volume; SW, stroke work; Ea, arterial elastance; Ees, end-systolic elastance; PVA, pressure-volume area; LV + S, left ventricle plus septum.
P < 0.05, HCR-M vs. LCR-M;
P < 0.05, HCR-F vs. LCR-F;
P < 0.05, HCR-M vs. HCR-F;
P < 0.05, LCR-M vs. LCR-F.
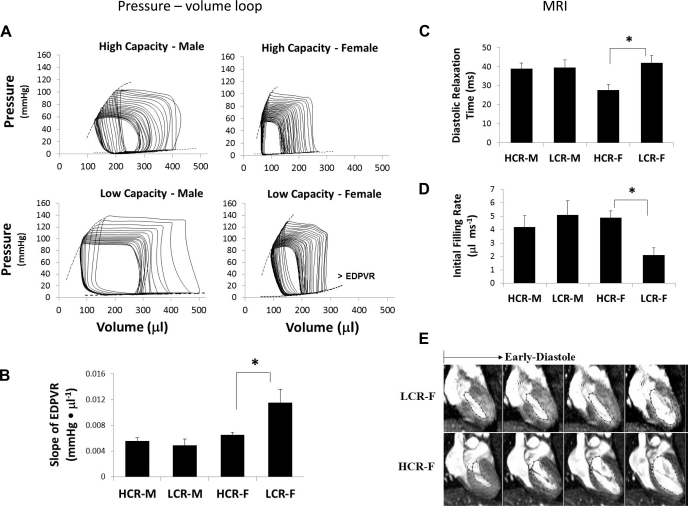
Pressure-volume analysis was performed under conditions of preload reduction in response to occlusion of the inferior vena cava to obtain load-independent indexes of systolic and diastolic function (Table 5 and Fig. 1). Among the systolic indexes, we detected a significant effect of sex on the relationship between dP/dtmax and EDV, suggesting an enhanced speed of contraction in females of both strains compared with males. That LCR-F exhibited the highest preload recruitable stroke work, slope of dP/dtmax vs. EDV, slope of PVA vs. EDV, and normalized maximum power (see above) suggests enhanced LV systolic contractility and stiffness. There was a significant sex effect with regard to the LV stiffness constant, i.e., the slope of the end-diastolic pressure-volume relationship (Table 5). Importantly, the LV of LCR-F exhibited increased diastolic stiffness as well as systolic stiffness compared with those of HCR-F, indicative of abnormalities of passive properties of ventricular relaxation as well as impairments in active properties of systolic contraction.
Table 5.
Load-independent systolic and diastolic indexes obtained by pressure-volume loop analysis in HCR-M, LCR-M, HCR-F, and LCR-F rats
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 8 | 12 | 8 | 12 | |
| Systolic indexes | |||||
| Ees quadratic, mmHg/μl | |||||
| Strain | 0.537 | 1.72 ± 0.28 | 2.19 ± 0.52 | 1.93 ± 0.36 | 1.90 ± 0.22 |
| Sex | 0.915 | ||||
| Interaction | 0.488 | ||||
| Preload recruitable SW | |||||
| Strain | 0.343 | 86 ± 8 | 87 ± 7 | 85 ± 6 | 97 ± 7 |
| Sex | 0.543 | ||||
| Interaction | 0.422 | ||||
| Slope of maximum dP/dt-EDV, mmHg·s−1·μl−1 | |||||
| Strain | 0.807 | 15.9 ± 3.12 | 12.7 ± 1.9d | 18.4 ± 1.7 | 20.5 ± 2.3 |
| Sex | 0.040 | ||||
| Interaction | 0.284 | ||||
| Slope of PVA-EDV, mmHg·μl−1·μl−1 | |||||
| Strain | 0.200 | 107 ± 8 | 109 ± 8 | 107 ± 8 | 128 ± 9 |
| Sex | 0.284 | ||||
| Interaction | 0.245 | ||||
| Diastolic indexes | |||||
| Slope of the end-diastolic pressure-volume relationship, mmHg/μl | |||||
| Strain | 0.146 | 0.0056 ± 0.0005 | 0.0049 ± 0.0010 | 0.0065 ± 0.0004 | 0.0115 ± 0.0021b |
| Sex | 0.014 | ||||
| Interaction | 0.057 | ||||
| span style='font-family:Symbol'>t (Weiss), ms | |||||
| Strain | 0.114 | 11.2 ± 0.5 | 12.5 ± 1.5 | 9.8 ± 0.4 | 11.3 ± 0.9 |
| Sex | 0.163 | ||||
| Interaction | 0.904 | ||||
| τ (Glantz), ms | |||||
| Strain | 0.963 | 14.5 ± 0.6 | 13.4 ± 0.9 | 13.3 ± 0.5 | 14.5 ± 1.0 |
| Sex | 0.981 | ||||
| Interaction | 0.186 | ||||
Values are means ± SE. τ, time constant of isovolumic relaxation.
P < 0.05, HCR-F vs. LCR-F;
P < 0.05, LCR-M vs. LCR-F.
Fig. 1.
Cardiac function parameters were investigated using pressure-volume loop analysis and cine MRI. A: representative pressure-volume loops with preload reduction. B: bar graph showing an increase in the end-diastolic pressure-volume relationship (EDPVR) in low-capacity runner (LCR) female (LCR-F) rats (n = 8–12 rats/group). LCR-M, LCR males; HCR, high-capacity runners; HCR-M, HCR males; HCR-F, HCR females. C and D: diastolic relaxation times (C) and initial filling rates (D) by cine MRI (n = 9–12 rats/group). E: representative long-axis cine MRI images showing left ventricular (LV) early diastole phases (frames 9–12 of 16 frames captured) in a cardiac cycle. LCR-F rats demonstrated delayed LV diastolic relaxation (top) compared with HCR-F rats (bottom). Red lines indicate LV cavity segmentations. All measured parameters are shown in Tables 4–6. *P < 0.05.
Cine MRI.
Cardiac MRI was performed on 23- to 24-wk-old rats (Table 6). BWs measured immediately before MRI scanning were elevated in LCR-F and LCR-M by 19.2% and 27.3% compared with their HCR counterparts, respectively (P < 0.05). Septal wall thickness was increased in LCR compared with HCR; however, when septal wall thickness was normalized to TL (TL determined at autopsy), no differences among the groups were observed. There was a significant effect of genotype on LV diastolic relaxation time, demonstrating prolonged diastolic relaxation time in LCR, an effect due largely to prolonged diastolic relaxation time in LCR-F rather than LCR-M. Indeed, paired comparisons indicated prolonged diastolic relaxation times in LCR-F compared with HCR-F. In concert with the prolonged diastolic relaxation time, there was a decrease in the initial, but not the peak, filling rate in LCR-F compared with HCR-F, suggesting that abnormal LV filling occurs during early diastole. In contrast, LCR-M showed no differences in diastolic relaxation time, initial filling rate, or peak filling rate compared with HCR-M. No differences were observed among groups in HR, EDV, end-systolic volume, stroke volume, cardiac output, CI, or ejection fraction.
Table 6.
Systolic and diastolic indexes in HCR-M, LCR-M, HCR-F, and LCR-F rats obtained by in vivo cine MRI
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 10 | 9 | 9 | 10 | |
| Age at MRI, wk | |||||
| Strain | 0.81 | 23.6 ± 0.04c | 23.7 ± 0.04 | 23.8 ± 0.03 | 23.7 ± 0.06 |
| Sex | 0.145 | ||||
| Interaction | 0.022 | ||||
| Septum thickness, mm | |||||
| Strain | 0.008 | 1.74 ± 0.004c | 1.88 ± 0.007d | 1.44 ± 0.005 | 1.62 ± 0.006b |
| Sex | <0.001 | ||||
| Interaction | 0.751 | ||||
| Septal wall thickness/TL, mm | |||||
| Strain | 0.055 | 4.25 ± 0.10 | 4.48 ± 0.18 | 3.99 ± 0.14 | 4.35 ± 0.156 |
| Sex | 0.217 | ||||
| Interaction | 0.654 | ||||
| Heart rate, beats/min | |||||
| Strain | 0.991 | 345 ± 12.9 | 342 ± 11.5 | 346 ± 12.9 | 349 ± 11.9 |
| Sex | 0.731 | ||||
| Interaction | 0.824 | ||||
| EDV, μl | |||||
| Strain | 0.434 | 562 ± 25.7c | 532 ± 21.2d | 377 ± 26.2 | 369 ± 19.7 |
| Sex | <0.001 | ||||
| Interaction | 0.645 | ||||
| End-systolic volume, μl | |||||
| Strain | 0.355 | 99 ± 10.1c | 83 ± 11.8d | 53 ± 5.6 | 53 ± 6.7 |
| Sex | <0.001 | ||||
| Interaction | 0.390 | ||||
| Stroke volume, μl | |||||
| Strain | 0.630 | 462 ± 24.4c | 449 ± 17.7d | 323 ± 24.3 | 316 ± 15.7 |
| Sex | <0.001 | ||||
| Interaction | 0.882 | ||||
| Systolic indexes | |||||
| Cardiac output, ml/min | |||||
| Strain | 0.634 | 161 ± 12.8c | 153 ± 7.3d | 112 ± 9.6 | 110 ± 6.5 |
| Sex | <0.001 | ||||
| Interaction | 0.754 | ||||
| Cardiac index, ml·min−1·g−1 | |||||
| Strain | 0.002 | 0.43 ± 0.030c | 0.33 ± 0.018a,d | 0.52 ± 0.032 | 0.43 ± 0.028b |
| Sex | 0.002 | ||||
| Interaction | 0.699 | ||||
| Ejection fraction, % | |||||
| Strain | 0.427 | 82.2 ± 1.67 | 84.5 ± 1.89 | 85.7 ± 1.28 | 86.0 ± 1.41 |
| Sex | 0.128 | ||||
| Interaction | 0.539 | ||||
| Diastolic indexes | |||||
| Diastolic relaxation time, ms | |||||
| Strain | 0.038 | 38.9 ± 3.05c | 39.6 ± 3.96 | 27.6 ± 3.06 | 42.1 ± 3.79b |
| Sex | 0.220 | ||||
| Interaction | 0.056 | ||||
| Peak filling rate, μl/ms | |||||
| Strain | 0.598 | 12.2 ± 1.05c | 11.8 ± 0.74d | 8.5 ± 0.84 | 8.1 ± 0.46 |
| Sex | <0.001 | ||||
| Interaction | 0.981 | ||||
| Initial filling rate, μl/ms | |||||
| Strain | 0.246 | 4.2 ± 0.85 | 5.1 ± 1.07d | 4.9 ± 0.50 | 2.1 ± 0.56b |
| Sex | 0.147 | ||||
| Interaction | 0.023 | ||||
Values are means ± SE.
P < 0.05, HCR-M vs. LCR-M;
P < 0.05, HCR-F vs. LCR-F;
P < 0.05, HCR-M vs. HCR-F;
P < 0.05, LCR-M vs. LCR-F.
Myocardial Remodeling
LVH.
Males of both strains had increased LV plus septum and right ventricular weights compared with females even when ventricular weight was normalized to TL (Table 7). Moreover, the relative weight of the LV plus septum of LCR-M was greater than that of HCR-M, demonstrating LV hypertrophy, a response that is consistent with higher blood pressure in LCR-M.
Table 7.
Ventricular weights normalized to TL in 30-wk-old HCR-M, LCR-M, HCR-F, and LCR-F rats
| Main Effect | P Value | HCR-M | LCR-M | HCR-F | LCR-F |
|---|---|---|---|---|---|
| Number of rats/group | 18 | 21 | 18 | 18 | |
| LV + S weight, mg | |||||
| Strain | 0.001 | 682 ± 15c | 801 ± 24a,d | 478 ± 10 | 521 ± 7 |
| Sex | 0.001 | ||||
| Interaction | 0.018 | ||||
| RV weight, mg | |||||
| Strain | 0.044 | 159 ± 5c | 179 ± 8a,d | 117 ± 5 | 119 ± 2 |
| Sex | 0.001 | ||||
| Interaction | 0.081 | ||||
| LV/TL, mg/g | |||||
| Strain | 0.003 | 16.9 ± 0.4c | 18.9 ± 0.5a,d | 13.5 ± 0.3 | 13.8 ± 0.2 |
| Sex | 0.001 | ||||
| Interaction | 0.039 | ||||
| RV/TL, mg/g | |||||
| Strain | 0.625 | 3.9 ± 0.1c | 4.2 ± 0.2d | 3.3 ± 0.2 | 3.1 ± 0.1 |
| Sex | 0.001 | ||||
| Interaction | 0.107 |
Values are means ± SE. Ages, body weights, TLs, and fat pad weights are shown in Table 1. RV, right venticle.
P < 0.05, HCR-M vs. LCR-M;
P < 0.05, HCR-M vs. HCR-F;
P < 0.05, LCR-M vs. LCR-F.
LV fibrosis.
LIGHT MICROSCOPY.
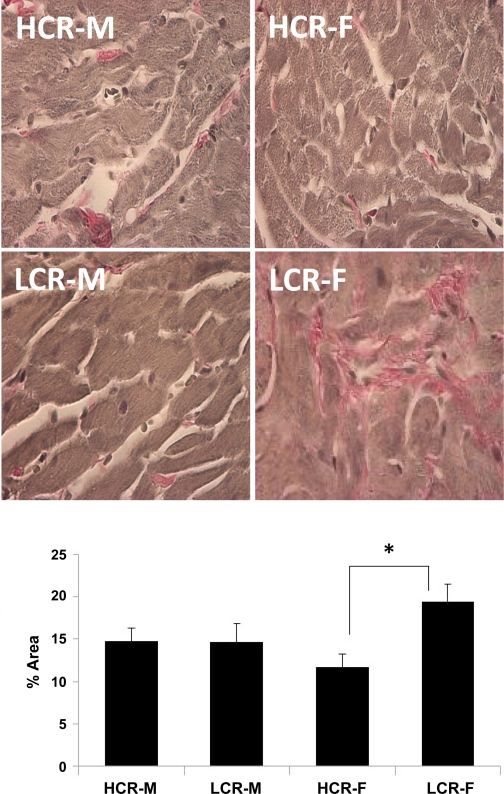
The relative amount of interstitial collagen (percent area) was elevated in the LCR strain compared with the HCR strain (P < 0.05). Collagen staining was 66% greater in LCR-F compared with HCR-F (P < 0.007; Fig. 2). Additionally, collagen staining was 32% greater in LCR-F compared with LCR-M but did not reach significance (P = 0.076). These results are consistent with the functional increase in passive LV stiffness in LCR-F compared with all other groups measured by pressure-volume loop analysis.
Fig. 2.
Top: representative light micrographs of Verhoeff van Gieson-stained sections of the LV showing pink-stained collagen fibers in the myocardial interstitium. Bottom: bar graph showing the results of a quantitative morphometric analysis indicating increased interstitial fibrosis in LCR-F compared with HCR-F (n = 5 rats/group). *P < 0.05.
TRANSMISSION ELECTRON MICROSCOPY.
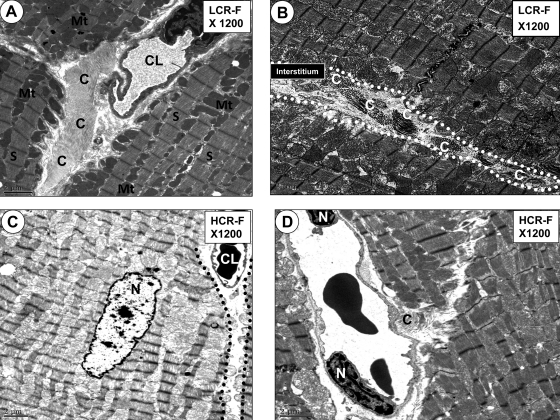
The LCR-F myocardium also exhibited dense pericapillary and interstitial bundles of collagen fibers (Fig. 3, A and C) compared with HCR-F (Fig. 3, B and D), results that are consistent with interstitial fibrosis observed at the light microscopic level in LCR-F.
Fig. 3.
Micrographs showing pericapillary and interstitial fibrosis in LCR-F. A: well-defined collagen bundles (C) constituting early interstitial pericapillary fibrosis in a LCR-F. Note the capillary lumen (CL) encircled by its endothelial cell and surrounded by the well-ordered collagen bundles within the myocardial interstitium. B: well-defined collagen bundles in the myocardial interstitium (outlined by the white dotted line) of a LCR-F. C: lack of any well-ordered collagen in the pericapillary interstitial regions of a HCR-F compared with the LCR-F in A and B. D: interstitial capillary in a HCR-F with only minimal collagen deposition in the pericapillary regions compared with the LCR-F in A and B. Mt, linear rows of mitochondria; S, sarcomere bounded by linear electron-dense Z-lines; N, cardiomyocyte nucleus. Magnification: ×1,200. Bars = 2 μm.
Mitochondrial Function
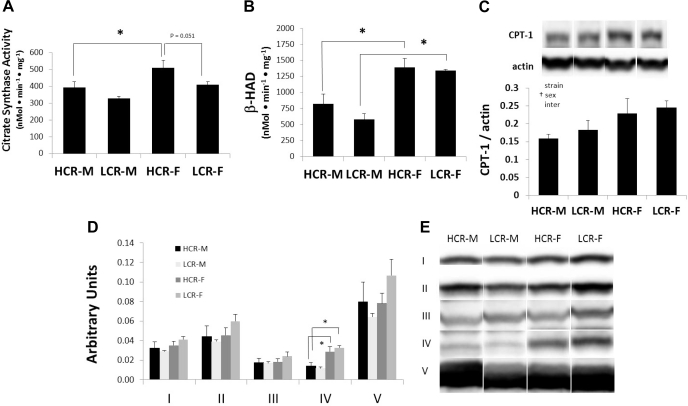
Citrate synthase activity/β-HAD.
There were strain and sex effects for mitochondrial citrate synthase activity and a sex effect for β-HAD activity (Fig. 4, A and B). Citrate synthase activity levels were elevated in HCR compared with LCR and in females compared with males. There was a trend suggesting decreased citrate synthase activity in LCR-F compared with HCR-F (P = 0.051). These data suggest that females have higher numbers of functional mitochondria than males and that HCR-F tend to have higher numbers of mitochondria than LCR-F as well as both groups of males. Females of both strains had elevated β-HAD activity compared with males, suggestive of higher FA oxidation in females. This result is consistent with higher voluntary activity levels in females as exercise is associated with elevated β-HAD in skeletal muscle (39).
Fig. 4.
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities (A and B), carnitine palmitoyltransferase 1 (CPT1; C), and oxidative phosphorylation (OXPHOS) complex I–V protein content (D and E) from LV protein extracts of HCR-M, HCR-F, LCR-M, and LCR-F (n = 5 rats/group). Representative Western blots of CPT1 and OXPHOS proteins are shown in C and E, respectively. *P < 0.05; †significant ANOVA main effect.
CPT1.
CPT1 is an outer mitochondrial membrane protein that catalyzes the esterification of long-chain FAs, thereby allowing for the their transport from the cytoplasm to the mitochondrion (33). There was a significant effect of sex on levels of CPT1, normalized to β-actin, in myocardial mitochondrial extracts, demonstrating elevated levels of CPT1 in females compared with males. No significant strain or interaction effects were detected.
OXPHOS.
Mitochondrial protein content of OXPHOS I, II, III, and V did not differ among groups; however, OXPHOS IV (complex IV, subunit 1) was elevated in females of both strains compared with their male counterparts (Fig. 4, D and E).
The AMPK/Sirt1 Axis
AMPK.
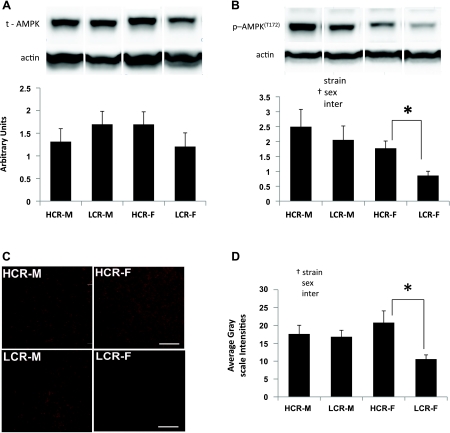
AMPK is a highly conserved serine/threonine kinase that monitors the energy state of the cell and coordinates the metabolic response in the energy-deprived failing heart (2). No differences were detected in total AMPK protein levels in LV whole cell protein extracts among the groups. There was a significant effect of sex on levels of p-AMPK(T172), demonstrating elevated activation of AMPK in the LV of males compared with females. Moreover, LCR-M hearts tended to have elevated p-AMPK(T172) levels compared with LCR-F (P = 0.056). Despite a trend suggesting lower p-AMPK(T172) in LCR-F compared with HCR-F, the ANOVA post hoc comparison did not achieve significance. On the other hand, using a separate t-test to compare p-AMPK(T172) only between HCR-F and LCR-F indicated reduced p-AMPK(T172) levels in LCR-F compared with HCR-F (t = 3.21, P = 0.01). These data suggest that a deficiency in AMPK signaling may contribute to the diastolic dysfunction in LCR-F. Indeed, AMPK deficiency exacerbates myocardial contractile dysfunction, and AMPK activation is considered as an adaptive mechanism in the transition from cardiac injury to heart failure (2, 46).
Sirt1.
Sirtuins are highly conserved class III histone deacetylases with homology to yeast silent information regulator 2. The role of Sirt1 in cardiac protection is now emerging (21, 44). Interestingly, beneficial effects of resveratrol, an activator of Sirt1, are attenuated in AMPK-deficient mice (47). Moreover, AMPK enhances Sirt1 activity by increasing cellular NAD+ levels, and thus there is an intimate association between AMPK- and Sirt1-mediated signaling (9). Sirt1 levels, as assessed by semiquantitative immunohistochemistry, indicated lower myocardial Sirt1 protein content in LCR compared with HCR rats (Fig. 5, C and D; strain effect, P < 0.05). We observed that, as in the case of AMPK, Sirt1 levels in LCR-F were half those observed in HCR-F, suggesting a deficiency in myocardial Sirt1 protein (P < 0.01). Regressing Sirt1 levels of HCR-F and LCR-F hearts with our index of fibrosis yielded a negative relationship [Sirt1 = 25.1 − (0.588% fibrosis), r2= 0.342, F= 4.15, P = 0.074, n = 10]; however, the relationship did not reach statistical significance. Nonetheless, the deficit in AMPK signaling and potentially in Sirt1 in LCR-F could play a major role in the diastolic dysfunction and fibrosis they display.
Fig. 5.
A and B: total (t) AMP-activated protein kinase (AMPK) and Thr172-phosphorylated AMPK [p-AMPK(T172)] content in myocardial protein extracts from the LV of HCR-M, HCR-F, LCR-M, and LCR-F. Representative bands are shown above the bar graphs. Values are means ± SE; n = 4 or 5 rats/group. C: representative micrographs showing sirtuin 1 (Sirt1) immunoflouresence in the LV. D: quantitation of the immunofluorescence signals (n = 5 rats/group). *P < 0.05; †significant ANOVA main effect.
DISCUSSION
While premenopausal females are normally at lesser risk of developing cardiovascular disease than men, the presence of metabolic syndrome negates this advantage (23, 37, 38). Furthermore, overweight and obese women tend to be at higher risk for the development of heart failure compared with overweight and obese men of similar age (27). Emerging evidence suggests that even young otherwise-healthy, overweight, and obese women exhibit subclinical systolic and diastolic dysfunction and concentric LV hypertrophy (38). These cardiac structural and functional abnormalities are associated with decreased whole body insulin sensitivity, increased myocardial FA uptake and oxidation, and decreased myocardial efficiency (37). Here, we present evidence suggesting that overweight inactive female rats (LCR), in the absence of comorbidities such as hypertension, baroreflex impairment, concentric LV hypertrophy, or cardiac steatosis, have increased LV diastolic stiffness and prolonged relaxation as well as increased arterial stiffness. Surprisingly, we observed little evidence of abnormalities in cardiac function in overweight and insulin-resistant LCR-M rats that had elevated blood pressure. Thus, the evidence suggests that intrinsic low aerobic capacity, overweightness, and female sex interact to promote diastolic dysfunction in rats.
As the female sex is normally associated with less risk and severity in the development of hypertension and cardiovascular disease (23, 24, 32), it is perhaps unsurprising that in this study LCR-F were protected from developing higher blood pressure, unlike their male counterparts. Although both male and female LCR displayed decreased baroreflex sensitivity (decreased reflex bradycardia), it is possible that the greater number of baroreflex sequences generated in females resulted in enough modulation of blood pressure fluctuations to maintain normal blood pressure levels in LCR-F. In LCR-M, in contrast, less baroreflex sensitivity and fewer baroreflex sequences generated resulted in less control of blood pressure fluctuations and higher blood pressure. Interestingly, diastolic dysfunction and LV interstitial fibrosis developed in LCR-F in the absence of hypertension, indicating that the mechanisms involved in the genesis of myocardial fibrosis in LCR-F may be intrinsic to the heart itself. Indeed, sex differences in fundamental cardiac function do exist (48), and estrogen modulates many myocardial properties (14, 29, 53).
Our data on mitochondrial FA oxidation suggest strain- and sex-related differences. The higher myocardial content of mitochondrial CPT1 and OXPHOS IV, in conjunction with higher citrate synthase and β-HAD activities, in the LVs of females suggests enhanced mitochondrial FA oxidation, oxidative enzyme function, and oxidative protein content. These results seem consistent with the functional pressure-volume data, indicating increased total mechanical energy generated per heartbeat in females compared with males. Finally, LCR-F hearts tended to have higher protein content of OXPHOS complex I–V proteins. Thus, our data suggest that delayed diastolic relaxation in LCR-F is not likely due to diminished mitochondrial oxidative function. CPT1 is an enzyme involved in mitochondrial FA uptake, and citrate synthase and β-HAD activities reflect FA oxidation. These observations suggest that in LCR-F rats, as in obese inactive women (34, 37, 38), there is increased myocardial uptake and utilization of FAs.
Importantly, LCR-F hearts displayed a reduction of the AMPK-Sirt1 signaling pathway compared with the other three groups (Fig. 5, A and B). It should be noted that these two molecules (AMPK and Sirt1) are fuel-sensing proteins that have coexisted in cells throughout evolution. A decline in Sirt1 protein levels is associated with increased interstitial fibrosis and a reduction in cardiac function in diabetic mice (44). Conversely, activation of Sirt1 by resveratrol is accompanied by reduced interstitial fibrosis and improved cardiac function in the LV of these mice (44). Thus, although our data are only indicative of a correlation, they suggest that the cardioprotective effects of AMPK and Sirt1, i.e., reduced interstitial fibrosis and improved diastolic relaxation, are attenuated in LCR-F hearts. Furthermore, since Sirt1 deficiency can contribute to reduced protein content of sarco(endo)plasmic Ca2+-ATPase 2, which is associated with delayed Ca2+ removal from the cytosol and diastolic dysfunction (44), this may also contribute to the abnormal diastolic function in Sirt1-deficient LCR-F.
Increases in arterial and ventricular stiffness tend to increase with age and can be associated with comorbidities such as hypertension, insulin resistance, and kidney disease, which further exacerbate cardiovascular disease (10, 25). Under normal circumstances, the stiffness (elastance) of the LV during contraction is matched to the stiffness of the systemic arterial vasculature (arterial-ventricular coupling) so that the heart functions efficiently to maintain a normal ejection fraction and cardiac reserve (6). Importantly, arterial stiffening can promote diastolic and systolic function by increasing the amount of mechanical work and myocardial O2 consumption required of the heart to perfuse body tissues, thus reducing the efficiency of pump function. Arterial-ventricular stiffening is common in patients with heart failure with preserved ejection fraction, and these patients tend to be older females with cardiorenal syndrome (obese hypertensives with kidney dysfunction). Although the female rats used in this study exhibited increased Ea, we did not detect arterial-ventricular uncoupling (a change in the ratio of Ea/end-systolic elastance). Normal ejection fraction was maintained in females and was associated with increases in systolic contractility, total mechanical work performed by the ventricle, increased FA oxidation, and total numbers of functional mitochondria compared with their male counterparts. The increase in total mechanical work was not detrimental to the LV in females as efficiency (efficiency = stroke work/PVA) was similar to that observed in males.
Limitations of the Study
This study was designed to investigate sex-related differences in cardiac function in the setting of obesity. The results of our study are somewhat unexpected and on the surface may have limited translational value absent any data on the role of endogenous estrogens or testosterone, blood lipids, glucose, and insulin. Most notably, our experimental design did not control for variations in the estrus cycle of female rats, which could affect circulating estrogen levels over a period of several days. Although testosterone levels are often viewed as constant in male rodents and humans, male testosterone levels are known to vary considerably throughout the day (17, 19, 23, 49). Thus, the concept of gating the levels of circulating steroids when comparing cardiac functions in control and impaired physiological states of male and female test subjects may be problematic. On the other hand, considerable care was taken to use cohorts of rats that had identical birth dates and procedures performed at identical ages. Thus, further study focusing on elucidating the causes of diastolic dysfunction in females and whether females are at greater risk for more serious heart disease is warranted. Our data on the AMPK/Sirt1 pathway, although interesting, should also be explored further to determine whether females are more sensitive to deficits in this pathway that would lead to more advanced heart disease.
Summary
Abnormalities in diastolic relaxation were demonstrated by both pressure loop and MRI measurements in LCR-F rats and occured in the absence of elevated blood pressure. These impairments are reminiscent of metabolic heart disease in obese women (23, 37, 38), where relaxation abnormalities often appear before the onset of systolic dysfunction. These relaxation abnormalities in LCR-F hearts likely reflect several biochemical and structural maladaptive alterations. The most obvious biochemical and structural abnormalities observed in the LV of LCR-F include a decreased AMPK/Sirt1 axis and increased interstitial fibrosis, respectively.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant 1-R01-HL-073101-02 (to J. R. Sowers) and by Veterans Affairs Merit System Grant 0018 (to J. R. Sowers). The LCR HCR rat model system was supported by NIH Grants R24-RR-017718 (to L. G. Koch and S. L. Britton) and RO1-DK-077200 (to S. L. Britton). The LCR and HCR models are available for collaborative study [contact S. L. Britton (brittons@umich.edu) or L. G. Koch (lgkoch@umich.edu)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.G.D. and J.R.S. conception and design of research; V.G.D., M.S.J., L.M., L.P., I.M., M.R.H., M.G., and W.C.K. performed experiments; V.G.D. and M.S.J. analyzed data; V.G.D., L.P., I.M., M.R.H., M.G., W.C.K., and J.R.S. interpreted results of experiments; V.G.D. and J.R.S. prepared figures; V.G.D., M.S.J., and J.R.S. drafted manuscript; V.G.D., M.S.J., L.M., L.P., I.M., M.R.H., M.G., W.C.K., S.L.B., L.G.K., and J.R.S. edited and revised manuscript; V.G.D., M.S.J., L.M., L.P., I.M., M.R.H., M.G., W.C.K., S.L.B., L.G.K., and J.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Terry L. Carmack and Lisa D. Watkinson of the Truman Memorial Veterans' Hospital Radiopharmaceutical Sciences Institute for surgical assistance. Rats were kindly provided by S. L. Britton and L. G. Koch. The authors acknowledge the support provided by the Veterans Affairs Biomolecular Imaging Center at the Harry S. Truman Veterans Affairs Hospital and the University of Missouri (Columbia, MO).
REFERENCES
- 1. Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969 [DOI] [PubMed] [Google Scholar]
- 2. Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res 90: 224–233, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985 [PubMed] [Google Scholar]
- 4. Bikman BT, Woodlief TL, Noland RC, Britton SL, Koch LG, Lust RM, Dohm GL, Cortright RN. High-fat diet induces Ikkβ and reduces insulin sensitivity in rats with low running capacity. Int J Sports Med 30: 631–635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics 28: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics 33: 100–109, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chantler PD, Nussbacher A, Gerstenblith G, Schulman SP, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Najjar SS. Abnormalities in arterial-ventricular coupling in older healthy persons are attenuated by sodium nitroprusside. Am J Physiol Heart Circ Physiol 300: H1914–H1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res 79: 269–278, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol 301: H2198–H2206, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN. Diastolic dysfunction: improved understanding using emerging imaging techniques. Am Heart J 160: 394–404, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol 33: 1345–1353, 2001 [DOI] [PubMed] [Google Scholar]
- 15. DeMarco VG, Johnson MS, Habibi J, Pulakat L, Gul R, Hayden MR, Tilmon R, Dellsperger KC, Winer N, Whaley-Connell AT, Sowers JR. Comparative analysis of telmisartan and olmesartan on cardiac function in the TG(mRen2)27 rat. Am J Physiol Heart Circ Physiol 300: H181–H190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR. Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr Hypertens Rep 12: 93–98, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Ellis GB, Desjardins C. Male rats secrete luteinizing hormone and testosterone episodically. Endocrinology 110: 1618–1627, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Emter CA, Bowles DK. Curing the cure: utilizing exercise to limit cardiotoxicity. Med Sci Sports Exerc 40: 806–807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta SK, Lindemulder EA, Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol 40: 731–738, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Habibi J, DeMarco VG, Ma L, Pulakat L, Rainey WE, Whaley-Connell AT, Sowers JR. Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression. Am J Physiol Heart Circ Physiol 300: H1484–H1491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122: 2170–2182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain SO, Barbato JC, Koch LG, Metting PJ, Britton SL. Cardiac function in rats selectively bred for low- and high-capacity running. Am J Physiol Regul Integr Comp Physiol 281: R1787–R1791, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 31: 17–22, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson MS, DeMarco VG, Heesch CM, Whaley-Connell AT, Schneider RI, Rehmer NT, Tilmon RD, Ferrario CM, Sowers JR. Sex differences in baroreflex sensitivity, heart rate variability, and end organ damage in the TGR(mRen2)27 rat. Am J Physiol Heart Circ Physiol 301: H1540–H1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev 7: 51–62, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Kass DA, Beyar R. Evaluation of contractile state by maximal ventricular power divided by the square of end-diastolic volume. Circulation 84: 1698–1708, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Koshman YE, Piano MR, Russell B, Schwertz DW. Signaling responses after exposure to 5α-dihydrotestosterone or 17β-estradiol in norepinephrine-induced hypertrophy of neonatal rat ventricular myocytes. J Appl Physiol 108: 686–696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kottam AT, Porterfield J, Raghavan K, Fernandez D, Feldman MD, Valvano JW, Pearce JA. Real time pressure-volume loops in mice using complex admittance: measurement and implications. Conf Proc IEEE Eng Med Biol Soc 1: 4336–4339, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res 101: 335–347, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Maris ME, Melchert RB, Joseph J, Kennedy RH. Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol 32: 35–39, 2005 [DOI] [PubMed] [Google Scholar]
- 33. McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev 5: 271–284, 1989 [DOI] [PubMed] [Google Scholar]
- 34. McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol 18: 421–429 and quiz 423–432, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris EM, Whaley-Connell AT, Thyfault JP, Britton SL, Koch LG, Wei Y, Ibdah JA, Sowers JR. Low aerobic capacity and high-fat diet contribute to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol 30: 112–119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 43: 1399–1404, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sowers JR, Whaley-Connel AT, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med 1: 5–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Srere PA. Citrate synthase. In: Methods Enzymol, edited by Lowenstein JM. New York: Academic Press, 1969, vol. 13, p. 3–11 [Google Scholar]
- 42. Suga H, Goto Y, Futaki S, Kawaguchi O, Yaku H, Hata K, Takasago T. Systolic pressure-volume area (PVA) as the energy of contraction in Starling's law of the heart. Heart Vessels 6: 65–70, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Suga H, Yamada O, Goto Y, Igarashi Y. Oxygen consumption and pressure-volume area of abnormal contractions in canine heart. Am J Physiol Heart Circ Physiol 246: H154–H160, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 298: H833–H843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 9: 592–606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59: 554–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to β-adrenergic stimulation. Am J Physiol Heart Circ Physiol 282: H256–H263, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Waite E, Kershaw Y, Spiga F, Lightman SL. A glucocorticoid sensitive biphasic rhythm of testosterone secretion. J Neuroendocrinol 21: 737–741, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario CM, Sowers JR. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, Link D, Ferrario C, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295: E103–E109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res 57: 388–394, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Zhou X, Ma L, Habibi J, Whaley-Connel AT, Hayden MR, Tilmon RD, Brown AN, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial tissue remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension 55: 880–888, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]