Abstract
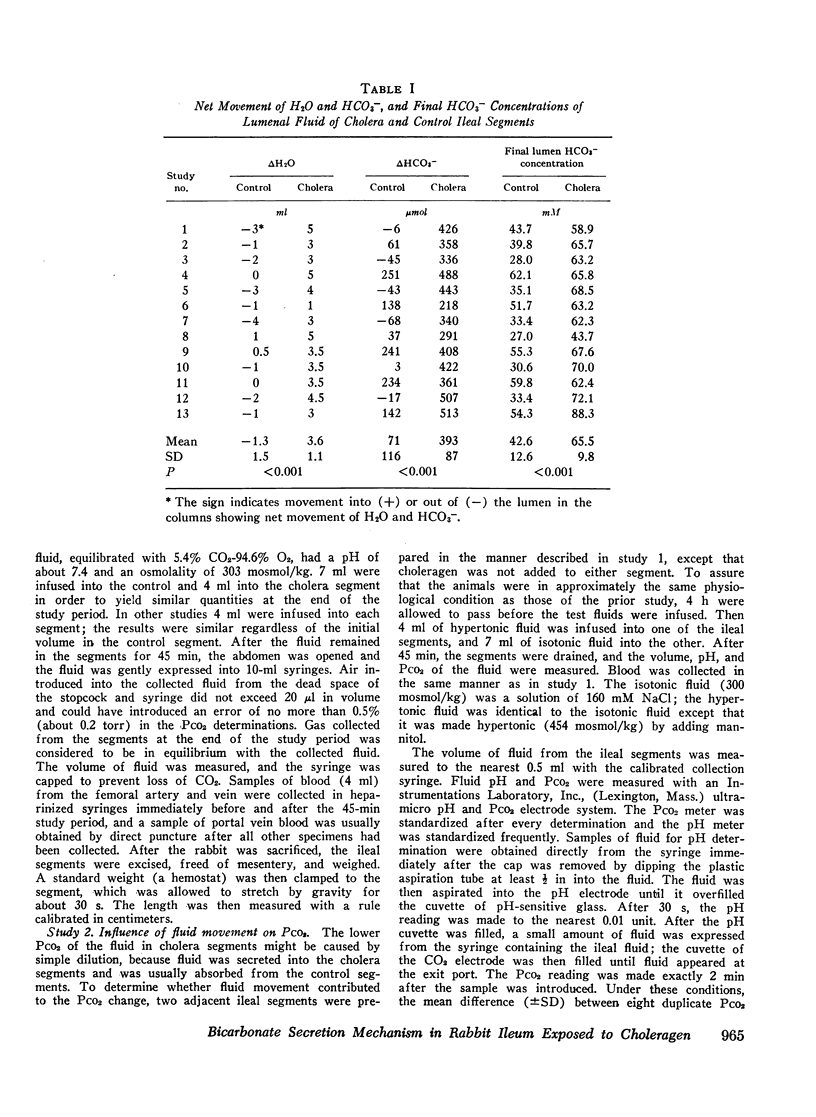
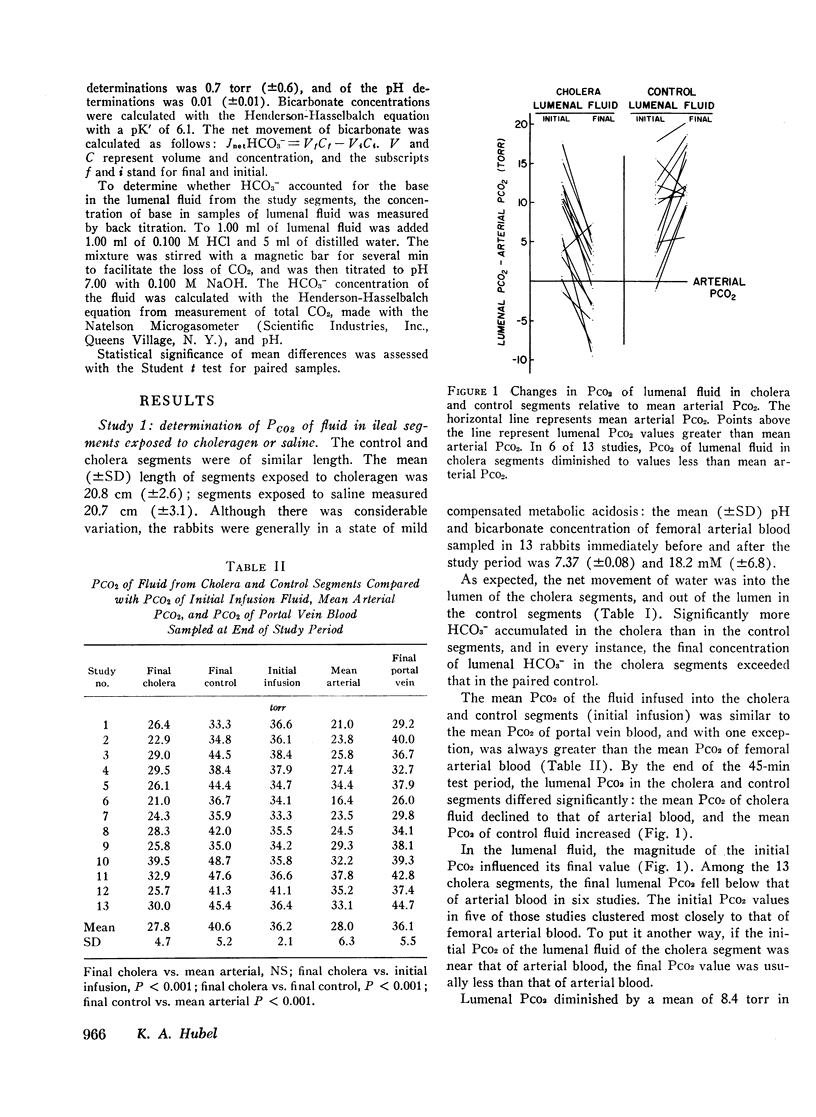
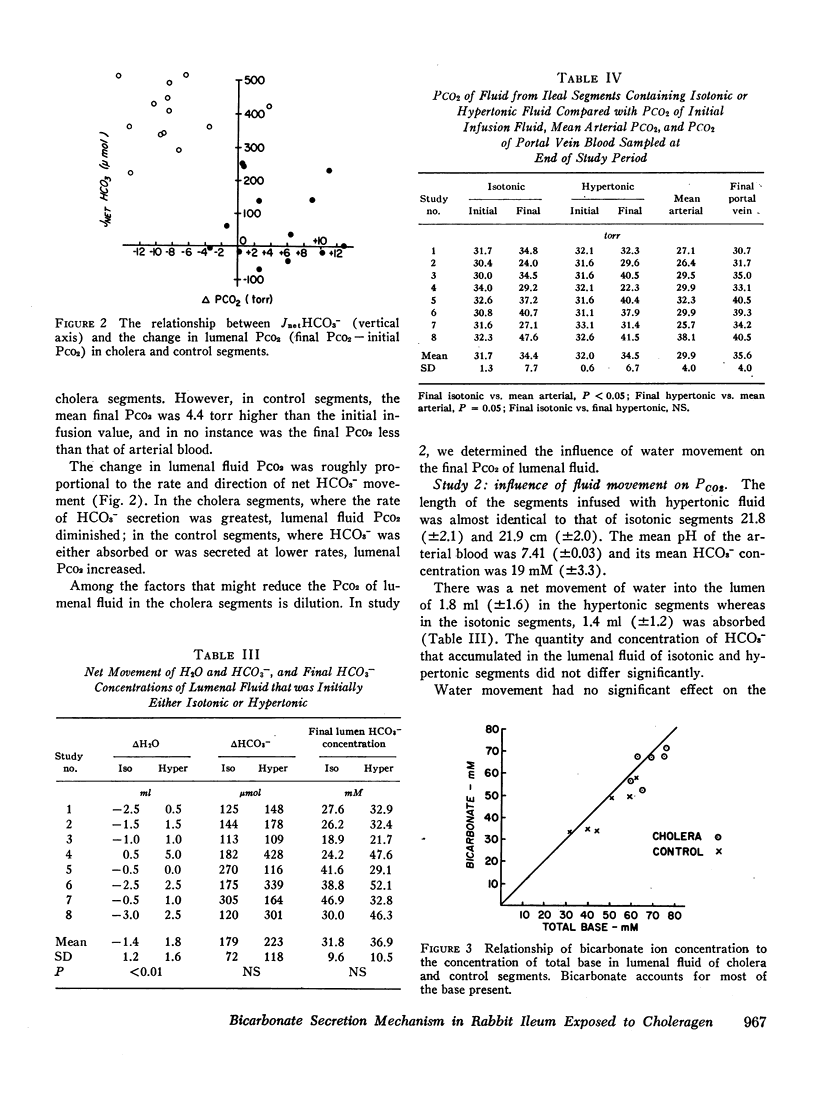
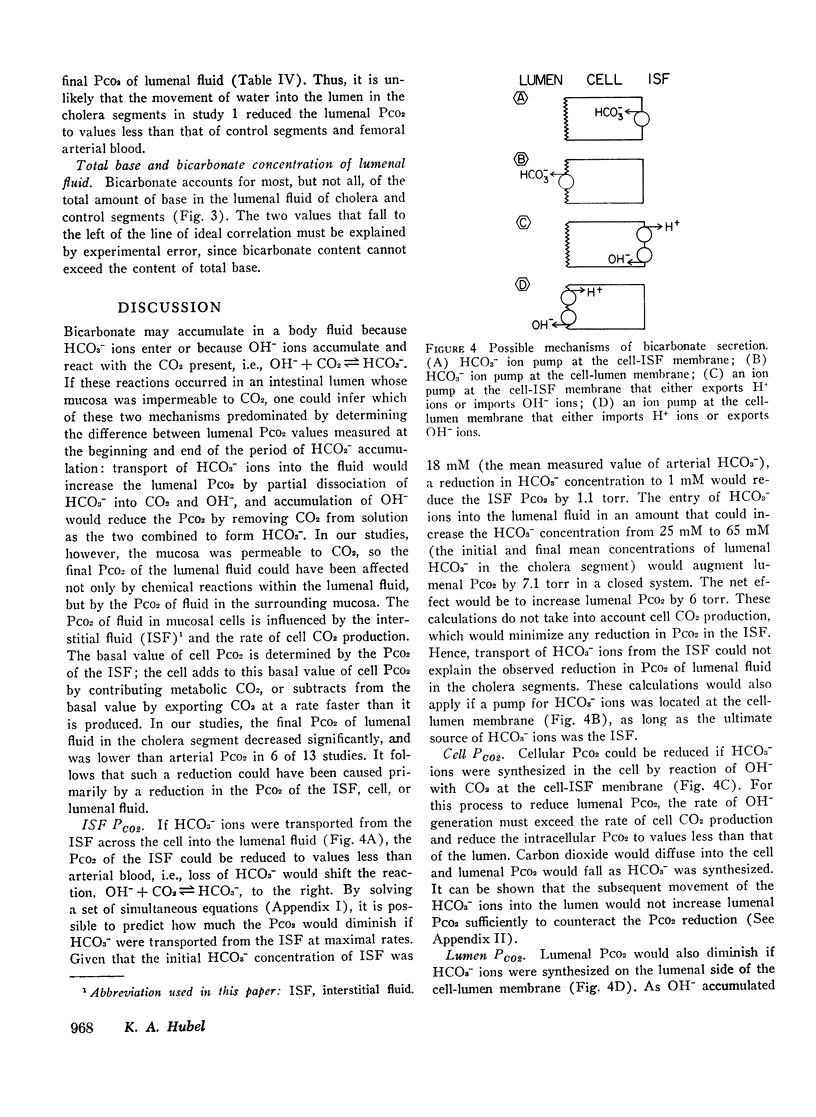
Bicarbonate may be secreted into the intestinal lumen in cholera because: HCO3− ions are transported, or because OH− ions accumulate and react with dissolved CO2 to form HCO3−. If HCO3− ions are transported into the lumen from the interstitial fluid, lumenal PCO2 should increase (HCO3− ⇌ OH− + CO2); if OH− accumulates, PCO2 should diminish. Net movement of H2O, and HCO3−, and changes in pH and PCO2 in lumenal fluid were studied in adjacent segments of rabbit ileum in vivo, one of which was exposed to choleragen. 4 h after exposure, segments were drained and infused with gassed Krebs-Henseleit solution whose PCO2 exceeded arterial PCO2. After 45 min, fluid was collected anaerobically from control and cholera segments. Among 13 cholera segments, lumenal PCO2 diminished by a mean of 8.4 torr and was less than femoral arterial blood in six instances. In the paired control segments, mean PCO2 increased by 4.4 torr, and was always greater than arterial PCO2. Dilution could not account for the low PCO2 in cholera segments because in hypertonic solutions that caused water to move into the lumen, the PCO2 did not differ from control values obtained with isotonic solutions. The results suggest that OH− accumulation (by addition of OH− or removal of H+) causes HCO3− secretion in cholera. This does not result from secretion of some other base (e.g., HPO4−), because HCO3− accounts for most of the base in the lumenal fluid. The PCO2 changes suggest that OH− reacts with CO2 at the cell-lumen interface, but reaction at the cell-interstitial fluid interface cannot be excluded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschke K., Bentzel C. J., Csáky T. Z. Asymmetry of osmotic flow in frog intestine: functional and structural correlation. Am J Physiol. 1970 Jun;218(6):1723–1731. doi: 10.1152/ajplegacy.1970.218.6.1723. [DOI] [PubMed] [Google Scholar]
- Roggin G. M., Banwell J. G., Yardley J. H., Hendrix T. R. Unimpaired response of rabbit jejunum to cholera toxin after selective damage to villus epithelium. Gastroenterology. 1972 Dec;63(6):981–989. [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]


