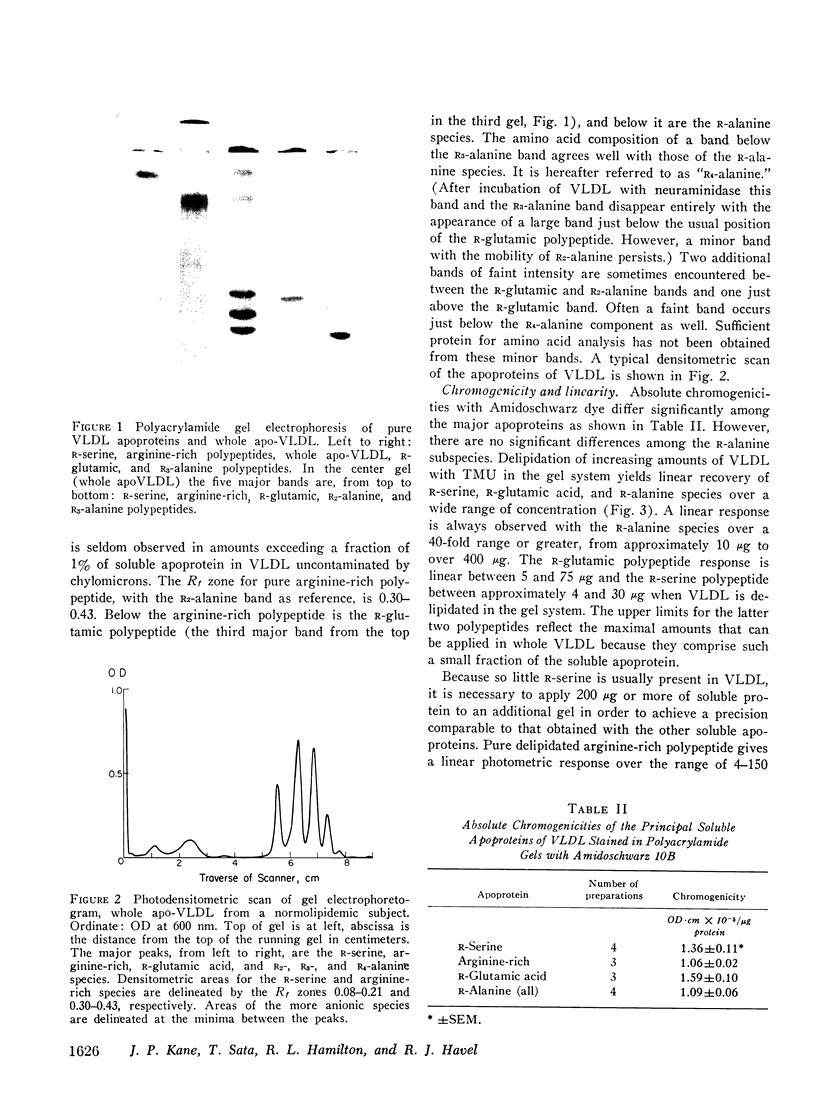
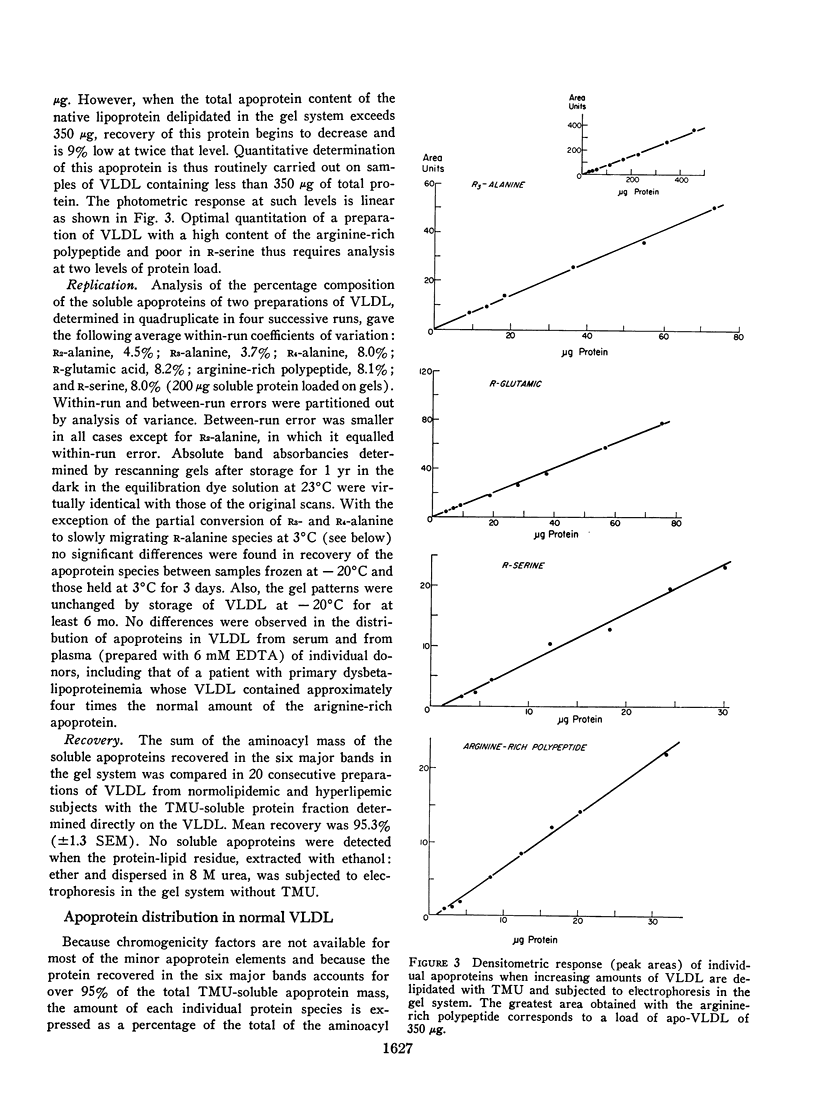
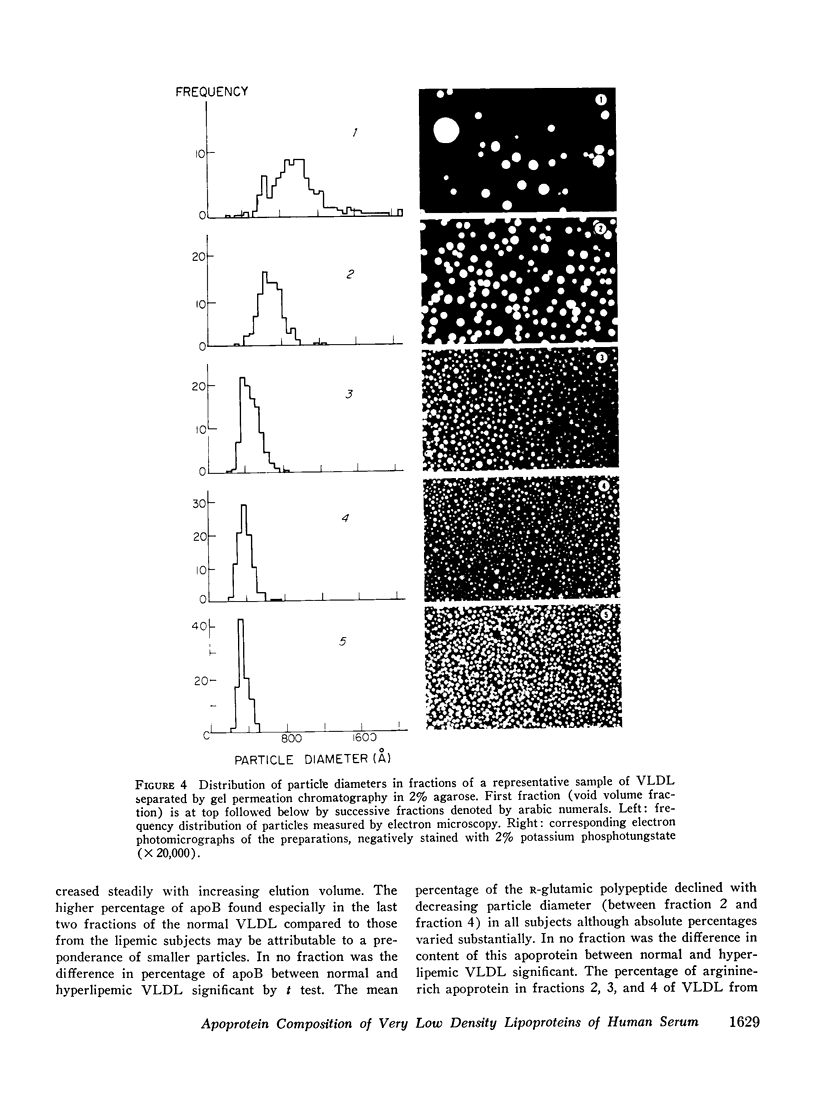
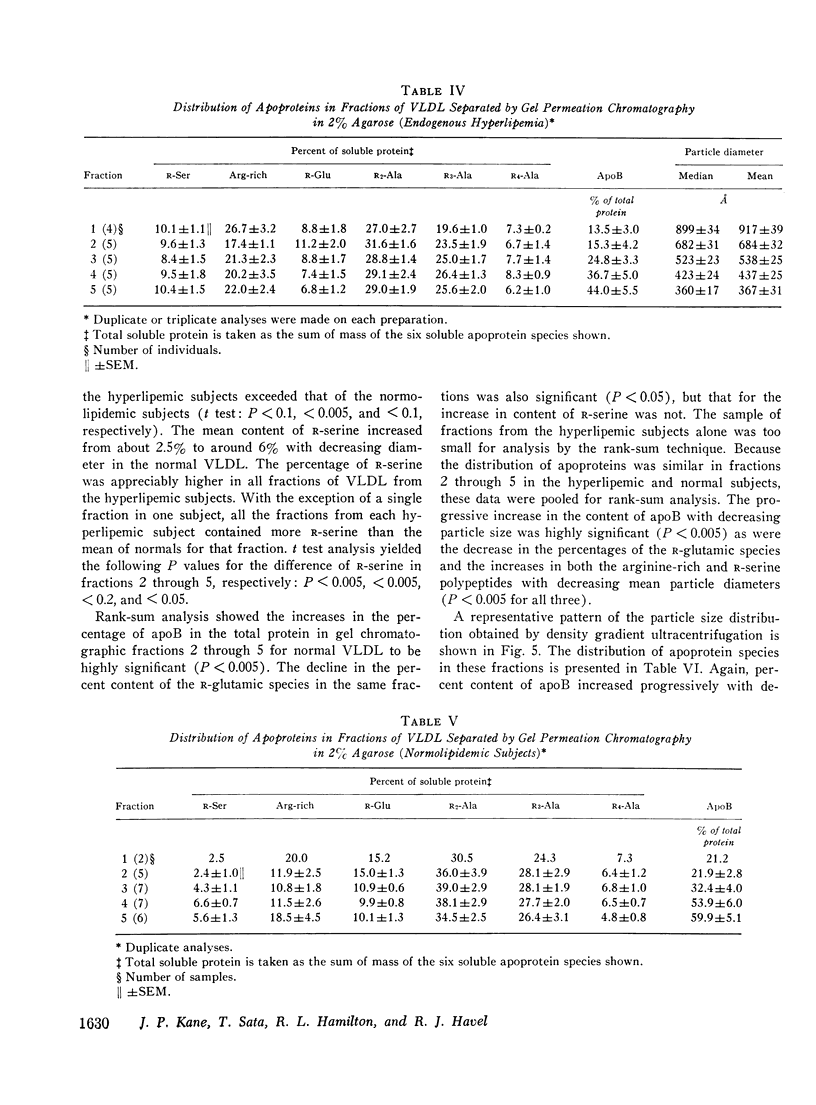
Abstract
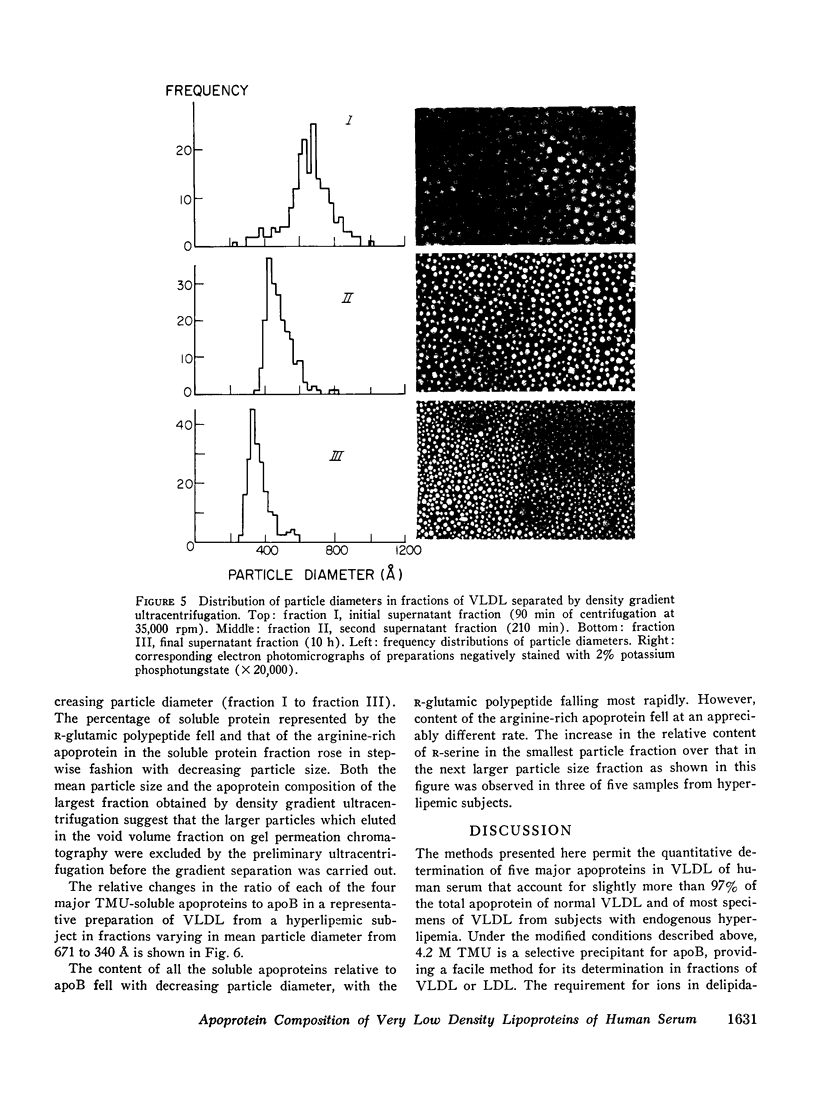
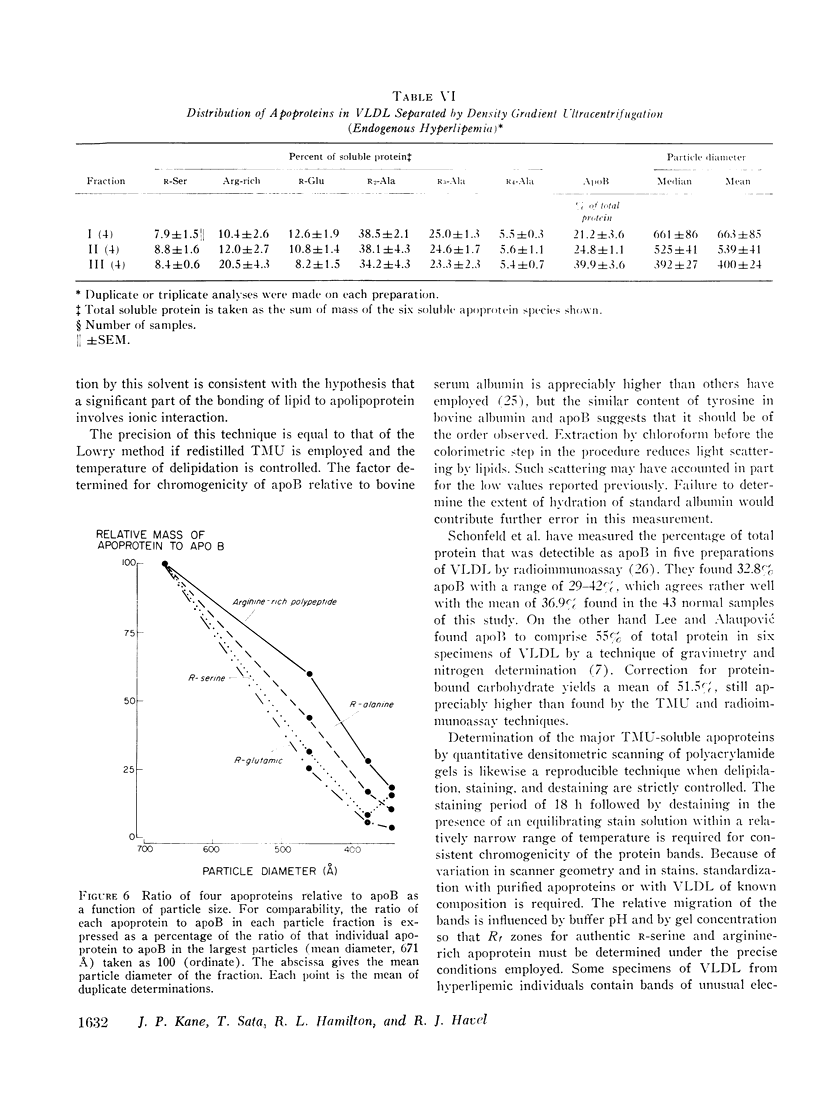
Methods for quantitation of the major apoproteins of human serum very low density lipoprotein have been developed employing tetramethylurea, which delipidates the lipoprotein and selectively precipitates apolipoprotein B. Six soluble apoproteins are separated by electrophoresis in polyacrylamide gel. One of these is a previously unrecognized species of R-alanine (R4-alanine), more anionic than the R3-alanine polypeptide. Conditions of staining have been found which yield reproducibly linear chromogenic response with native lipoprotein and with each purified apoprotein. Recovery of protein in the seven species measured accounts for over 97% of the total in the very low density lipoprotein of normolipidemic individuals and in most samples from individuals with endogenous hyperlipemia. The mean content of apolipoprotein B in 43 samples from normolipidemic subjects was 36.9(+/-1.2 SEM)% of total protein, The distribution of the major soluble apoproteins as mean (+/-SEM) percentage of the soluble fraction was : R-serine, 5.3+/-o.5; arginine-rich, 20.6+/-1.0; R-glutamic, 10.6+/-0.4; R2-alanine, 28.3+/-0.7; R3-alanine, 26.9+/-0.5; and R4-alanine, 8.0+/-0.5. Distribution of the apoproteins was a function of particle diameter of very low density lipoprotein in fractions separated by gel permeation chromatography and by density gradient ultracentrifugation. In fractions below 700-800 A, apolipoprotein B comprised an increasing percentage of the total protein with decreasing particle diameter. Among the soluble proteins the percentage of the arginine-rich and R-serine polypeptides increased and that of the R-glutamic polypeptide declined progressively with decreasing particle size. Apoprotein distribution was similar in fractions of similar particle size from normolipidemic and hyperlipemic subjects with the exception that all fractions from the hyperlipemic subjects contained more R-serine and some, more arginine rich polypeptide. Even in the absence of chylomicrons, the distribution of soluble apoproteins in particles of diameters greater than 700-800 A was usually similar to that of the smallest particles. This suggests that the largest particles may include products of the partial catabolism of chylomicrons.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYRAULT-JARRIER M., CHEFTEL R. I., POLONOVSKI J. [The glucides of the beta-lipoprotein with a Sf of 1.063 between 0 and 12 in human blood serum]. Bull Soc Chim Biol (Paris) 1961;43:811–816. [PubMed] [Google Scholar]
- Brewer H. B., Jr, Shulman R., Herbert P., Ronan R., Wehrly K. The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins. J Biol Chem. 1974 Aug 10;249(15):4975–4984. [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Further separation of the apoproteins of the human plasma very low density lipoproteins. Biochim Biophys Acta. 1970 Mar 31;200(3):573–575. doi: 10.1016/0005-2795(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- Eisenberg S., Bilheimer D., Lindgren F., Levy R. I. On the apoprotein composition of human plasma very low density lipoprotein subfractions. Biochim Biophys Acta. 1972 Feb 21;260(2):329–333. doi: 10.1016/0005-2760(72)90045-8. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Brown W. V., Levy R. I., Birnbaumer M. E., Fredrickson D. S. Evidence for the identity of the major apoprotein in low density and very low density lipoproteins in normal subjects and patients with familial hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1486–1494. doi: 10.1172/JCI106945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Havel R. J., Kane J. P., Blaurock A. E., Sata T. Cholestasis: lamellar structure of the abnormal human serum lipoprotein. Science. 1971 Apr 30;172(3982):475–478. doi: 10.1126/science.172.3982.475. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P. Primary dysbetalipoproteinemia: predominance of a specific apoprotein species in triglyceride-rich lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2015–2019. doi: 10.1073/pnas.70.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med. 1969;15:117–154. [PubMed] [Google Scholar]
- Havel R. J., Shore V. G., Shore B., Bier D. M. Role of specific glycopeptides of human serum lipoproteins in the activation of lipoprotein lipase. Circ Res. 1970 Oct;27(4):595–600. doi: 10.1161/01.res.27.4.595. [DOI] [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Richards E. G., Havel R. J. Subunit heterogeneity in human serum beta lipoprotein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1075–1082. doi: 10.1073/pnas.66.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee D. M., Alaupović P. Composition and concentration of apolipoproteins in very-low-and low-density lipoproteins of normal human plasma. Atherosclerosis. 1974 May-Jun;19(3):501–520. [PubMed] [Google Scholar]
- MARSHALL W. E., KUMMEROW F. A. The carbohydrate constituents of human serum beta-lipoprotein: galactose, mannose, glucosamine and sialic acid. Arch Biochem Biophys. 1962 Aug;98:271–273. doi: 10.1016/0003-9861(62)90183-2. [DOI] [PubMed] [Google Scholar]
- Margolis S., Langdon R. G. Studies on human serum beta-1-lipoprotein. I. Amino acid composition. J Biol Chem. 1966 Jan 25;241(2):469–476. [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A. G., Van den Hamer C. J., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal D-galactose residues. J Biol Chem. 1966 Aug 25;241(16):3745–3749. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Sata T., Havel R. J., Jones A. L. Characterization of subfractions of triglyceride-rich lipoproteins separated by gel chromatography from blood plasma of normolipemic and hyperlipemic humans. J Lipid Res. 1972 Nov;13(6):757–768. [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Lees R. S., George P. K., Pfleger B. Assay of total plasma apolipoprotein B concentration in human subjects. J Clin Invest. 1974 May;53(5):1458–1467. doi: 10.1172/JCI107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore B., Shore V. Isolation and characterization of polypeptides of human serum lipoproteins. Biochemistry. 1969 Nov;8(11):4510–4516. doi: 10.1021/bi00839a043. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry. 1973 Jan 30;12(3):502–507. doi: 10.1021/bi00727a022. [DOI] [PubMed] [Google Scholar]





