Abstract
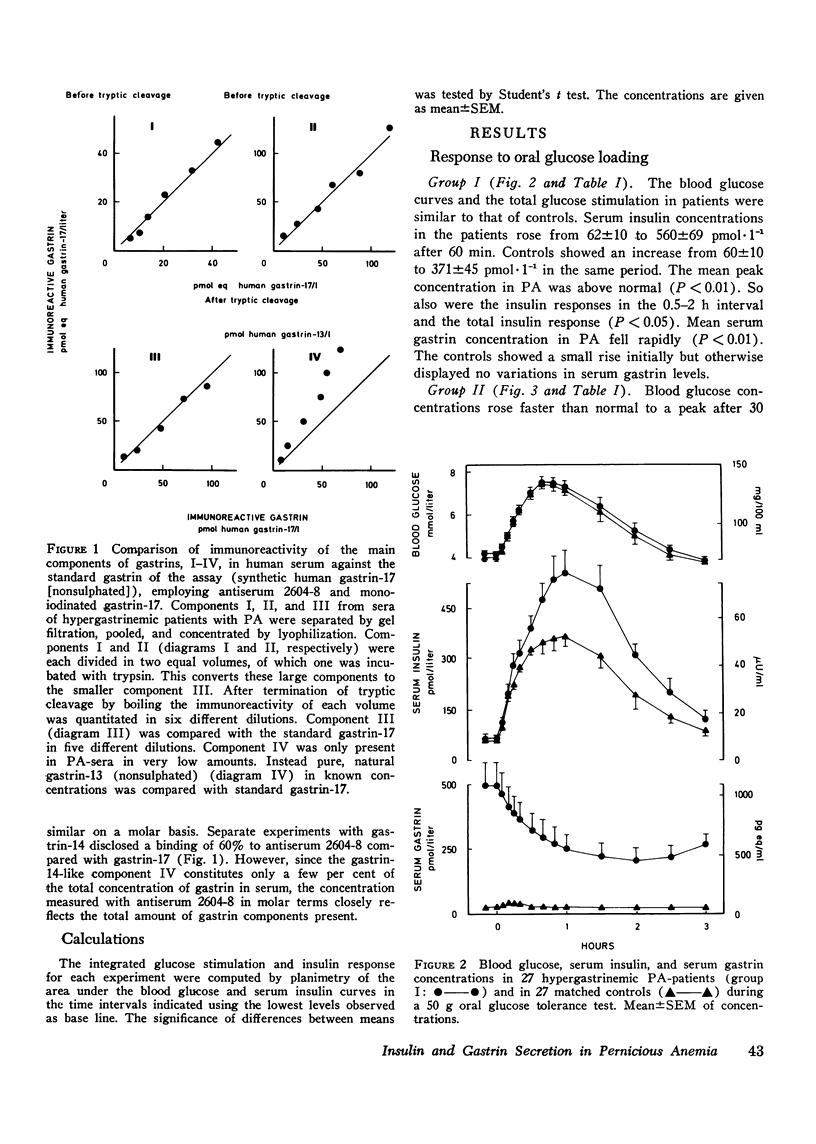
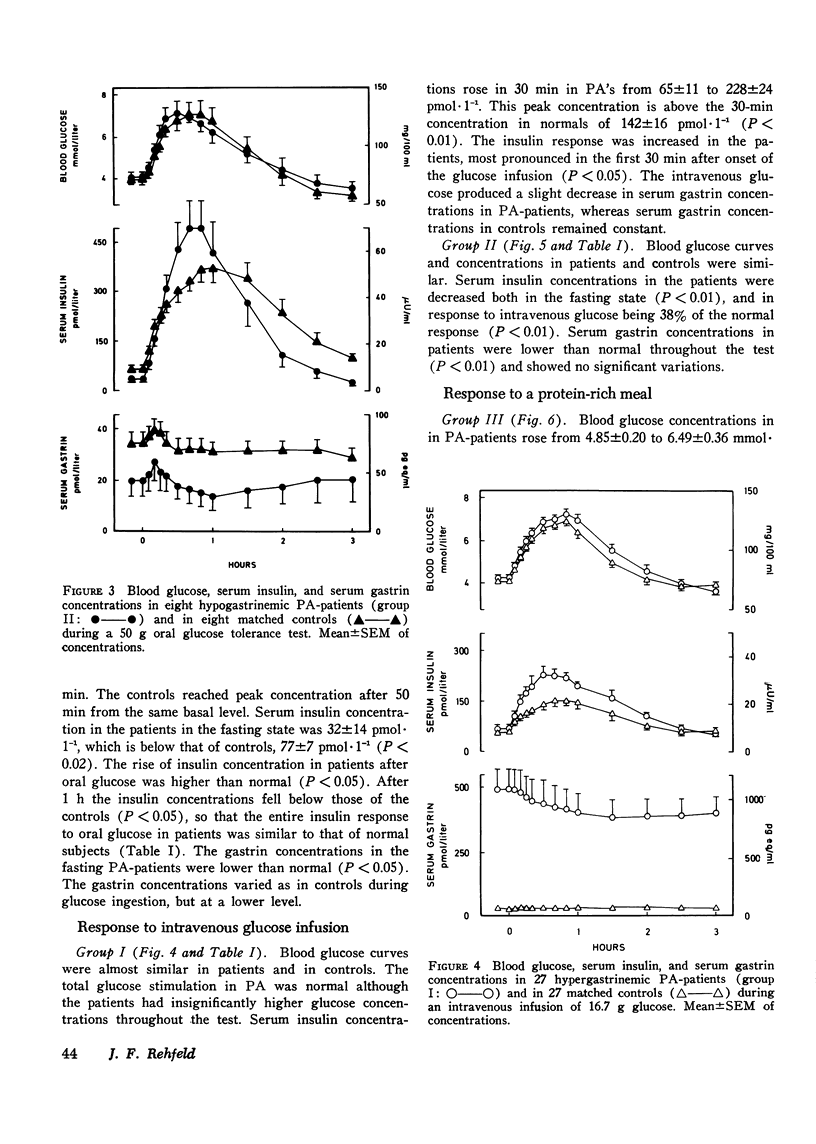
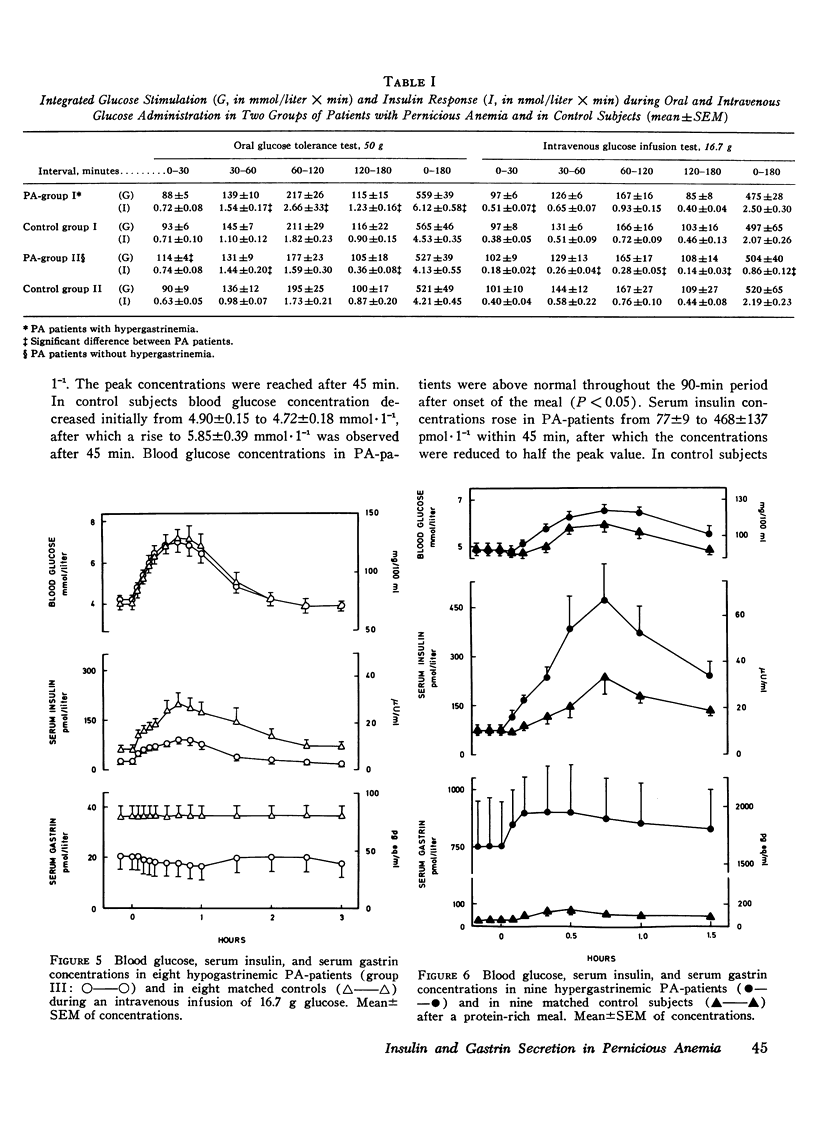
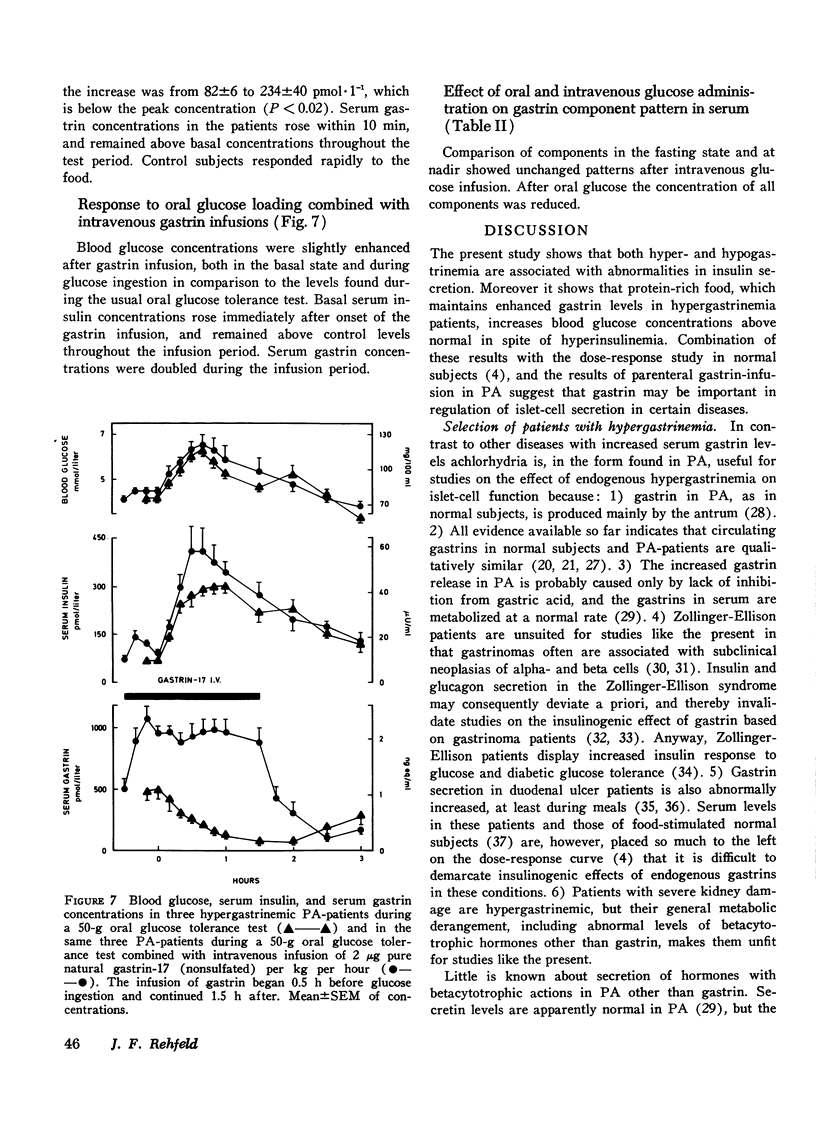
The insulin and gastrin response to oral glucose, intravenous glucose, or a protein-rich meal were measured in 44 nondiabetic patients with pernicious anemia (PA) and in 44 control subjects. 36 of the PA-patients had hypergastrinemia, while serum gastrin concentrations in the remaining eight patients were below normal. Three hypergastrinemic PA-patients were in addition studied during an oral glucose loading with synchronous intravenous infusion of gastrin-17. During both oral and intravenous glucose tests blood glucose concentrations were similar in patients and in controls. After ingestion of protein blood glucose concentrations in PA-patients with hypergastrinemia were above those of the controls (P less than 0.05). Parenteral infusion of gastrin-17 during oral glucose loading also increased blood glucose concentrations above the levels observed after glucose alone. In PA-patients with hypergastrinemia the insulin response was augmented in all tests. In patients with hypogastrinemia serum insulin concentrations were lower than normal in the fasting state and during stimulation with glucose intravenously (P less than 0.01). In hypergastrinemic patients serum gastrin concentrations decreased after oral as well as intravenous glucose administration. The decrease was larger during the oral test. In hypogastrinemia oral glucose induced, as in controls, a small initial rise followed by a slow fall in serum gastrin concentrations. No variations were seen in these patients during the intravenous glucose infusion. Gel filtration of serum from hypergastrinemic patients disclosed a decrease in the concentrations of all four main components of gastrin during the glucose loadings. Taken together with earlier studies on the effect of exogenous gastrin the results suggest that endogenous hypergastrinemia induces hyperglycemia and potentiates insulin secretion. In contrast hypogastrinemia is associated with hypoinsulinism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan K. D., Vance J. E., Williams R. H. Insulin and glucagon release from isolated islets of Langerhans. Effect of enteric factors. Diabetes. 1969 Jun;18(6):381–386. doi: 10.2337/diab.18.6.381. [DOI] [PubMed] [Google Scholar]
- Budillon G., Mazzacca G., Squame G. Failure of endogenous gastrin release to affect serum insulin. Digestion. 1973;8(3):201–207. doi: 10.1159/000197315. [DOI] [PubMed] [Google Scholar]
- Cataland S., Crockett S. E., Brown J. C., Mazzaferri E. L. Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab. 1974 Aug;39(2):223–228. doi: 10.1210/jcem-39-2-223. [DOI] [PubMed] [Google Scholar]
- Dupre J., Curtis J. D., Unger R. H., Waddell R. W., Beck J. C. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969 Apr;48(4):745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S., Walsh J. H. Gastric acid secretion rate and buffer content of the stomach after eating. Results in normal subjects and in patients with duodenal ulcer. J Clin Invest. 1973 Mar;52(3):645–657. doi: 10.1172/JCI107226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two minigastrins from Zollinger-Ellison tumour tissue. Gut. 1974 Sep;15(9):683–685. doi: 10.1136/gut.15.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. R., Ardill J., Buchanan K. D. Gastrin and insulin release. Diabetologia. 1975 Feb;11(1):89–92. doi: 10.1007/BF00422824. [DOI] [PubMed] [Google Scholar]
- Hinz M., Katsilambros N., Schweitzer B., Raptis S., Pfeiffer E. F. The role of the exocrine pancreas in the stimulation of insulin secretion by intestinal hormones. I. The effect of pancreozymin, secretin, gastrin-pentapeptide and of glucagon upon insulin secretion of isolated islets of rat pancreas. Diabetologia. 1971 Feb;7(1):1–5. doi: 10.1007/BF02346246. [DOI] [PubMed] [Google Scholar]
- Howitz J., Rehfeld J. F. Letter: Serum-gastrin, vitiligo, and achlorhydric atrophic gastritis. Lancet. 1974 Dec 7;2(7893):1399–1400. doi: 10.1016/s0140-6736(74)92281-8. [DOI] [PubMed] [Google Scholar]
- Howitz J., Rehfeld J. F. Serum-gastrin in vitiligo. Lancet. 1974 May 4;1(7862):831–833. doi: 10.1016/s0140-6736(74)90483-8. [DOI] [PubMed] [Google Scholar]
- Irvine W. J., Clarke B. F., Scarth L., Cullen D. R., Duncan L. J. Thyroid and gastric autoimmunity in patients with diabetes mellitus. Lancet. 1970 Jul 25;2(7665):163–168. doi: 10.1016/s0140-6736(70)92531-6. [DOI] [PubMed] [Google Scholar]
- Iversen J. Secretion of glucagon from the isolated, perfused canine pancreas. J Clin Invest. 1971 Oct;50(10):2123–2136. doi: 10.1172/JCI106706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto A., Mizuno Y., Tasaka Y., Kosaka K. Stimulation of glucagon secretion by tetragastrin. Endocrinology. 1970 May;86(5):1175–1180. doi: 10.1210/endo-86-5-1175. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M. Insulin secretion in normal and diabetic individuals. Adv Intern Med. 1970;16:103–134. [PubMed] [Google Scholar]
- Larsson L. I., Grimelius L., Håkanson R., Rehfeld J. F., Stadil F., Holst J., Angervall L., Sundler F. Mixed endocrine pancreatic tumors producing several peptide hormones. Am J Pathol. 1975 May;79(2):271–284. [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Ljungberg O., Sundler F., Håkanson R., Svensson S. O., Rehfeld J., Stadil R., Holst J. Antor-pyloric gastrinoma associated with pancreatic nesidioblastosis and proliferation of islets. Virchows Arch A Pathol Pathol Anat. 1973;360(4):305–314. doi: 10.1007/BF00548351. [DOI] [PubMed] [Google Scholar]
- MOORE J. M., NEILSON J. M. ANTIBODIES TO GASTRIC MUCOSA AND THYROID IN DIABETES MELLITUS. Lancet. 1963 Sep 28;2(7309):645–647. doi: 10.1016/s0140-6736(63)90448-3. [DOI] [PubMed] [Google Scholar]
- MacCuish A. C., Irvine W. J., Barnes E. W., Duncan L. J. Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet. 1974 Dec 28;2(7896):1529–1531. doi: 10.1016/s0140-6736(74)90281-5. [DOI] [PubMed] [Google Scholar]
- McGuigan J. E., Trudeau W. L. Differences in rates of gastrin release in normal persons and patients with duodenal-ulcer disease. N Engl J Med. 1973 Jan 11;288(2):64–66. doi: 10.1056/NEJM197301112880202. [DOI] [PubMed] [Google Scholar]
- Munichoodappa C., Kozak G. P. Diabetes mellitus and pernicious anemia. Diabetes. 1970 Oct;19(10):719–722. doi: 10.2337/diab.19.10.719. [DOI] [PubMed] [Google Scholar]
- Nerup J., Andersen O. O., Bendixen G., Egeberg J., Poulsen J. E. Anti-pancreatic cellular hypersensitivity in diabetes mellitus. Diabetes. 1971 Jun;20(6):424–427. doi: 10.2337/diab.20.6.424. [DOI] [PubMed] [Google Scholar]
- Ohgawara H., Mizuno Y., Tasaka Y., Kosaka K. Effect of the C-terminal tetrapeptide amide of gastrin on insulin secretion in man. J Clin Endocrinol Metab. 1969 Sep;29(9):1261–1262. doi: 10.1210/jcem-29-9-1261. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehefeld J. F. Three components of gastrin in human serum. Gel filtration studies on the molecular size of immunoreactive serum gastrin. Biochim Biophys Acta. 1972 Dec 28;285(2):364–372. [PubMed] [Google Scholar]
- Rehfeld J. F. Effect of gastrin and its C-terminal tetrapeptide on insulin secretion in man. Acta Endocrinol (Copenh) 1971 Jan;66(1):169–176. doi: 10.1530/acta.0.0660169. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Gastrins in serum. A review of gastrin radioimmunoanalysis and the discovery of gastrin heterogeneity in serum. Scand J Gastroenterol. 1973;8(7):577–583. [PubMed] [Google Scholar]
- Rehfeld J. F., Hippe E. Serum insulin response to oral glucose in pernicious anemia. Scand J Gastroenterol. 1970;5(8):713–718. [PubMed] [Google Scholar]
- Rehfeld J. F., Lauritsen K. B., Stadil F. Insulin secretion in the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl. 1976;37:63–66. [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Baden H., Fischerman K. The enteral insulin-stimulation after Whipple's operation. Diabetologia. 1975 Jun;11(3):207–210. doi: 10.1007/BF00422323. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Rubin B. Production and evaluation of antibodies for the radioimmunoassay of gastrin. Scand J Clin Lab Invest. 1972 Oct;30(2):221–232. doi: 10.3109/00365517209081114. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F. The effect of gastrin on basal- and glucose-stimulated insulin secretion in man. J Clin Invest. 1973 Jun;52(6):1415–1426. doi: 10.1172/JCI107315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F. The glucose-induced gastrointestinal stimulation of insulin secretion in man: relation to age and to gastrin release. Eur J Clin Invest. 1975 Jun 12;5(3):273–283. doi: 10.1111/j.1365-2362.1975.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Vikelsoe J. Immunoreactive gastrin components in human serum. Gut. 1974 Feb;15(2):102–111. doi: 10.1136/gut.15.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDBERG A., GRONBERG A. Diabetes mellitus and pernicious anaemia. Acta Med Scand. 1960 Feb 17;166:147–150. doi: 10.1111/j.0954-6820.1960.tb17365.x. [DOI] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F., Christiansen L. A., Malmström Patterns of gastrin components in serum during feeding in normal subjects and duodenal ulcer patients. Scand J Gastroenterol. 1975;10(8):863–868. [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8(2):101–112. [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Preparation of 125 I-labelled synthetic human gastrin I for radioimmunoanalysis. Scand J Clin Lab Invest. 1972 Dec;30(4):361–368. doi: 10.3109/00365517209080271. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Further studies on the nature of immunoreactive gastrin in human plasma. Gastroenterology. 1971 Feb;60(2):203–214. [PubMed] [Google Scholar]


