Abstract
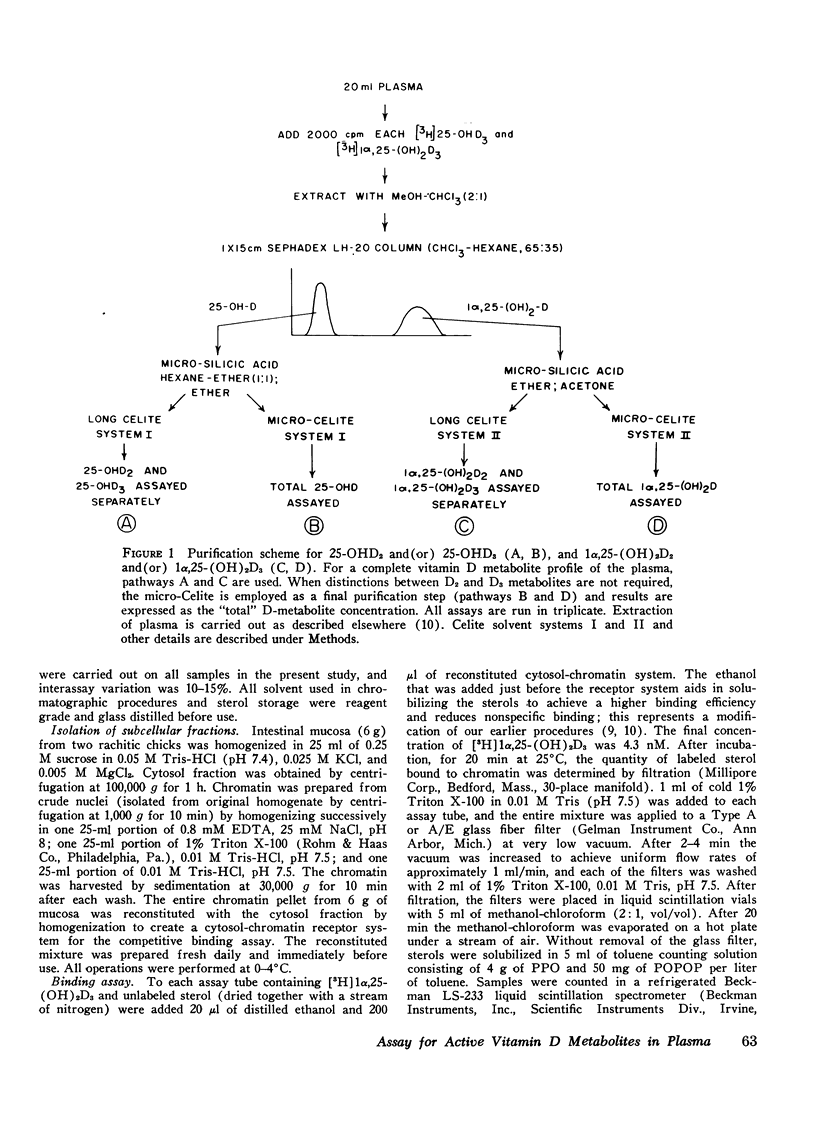
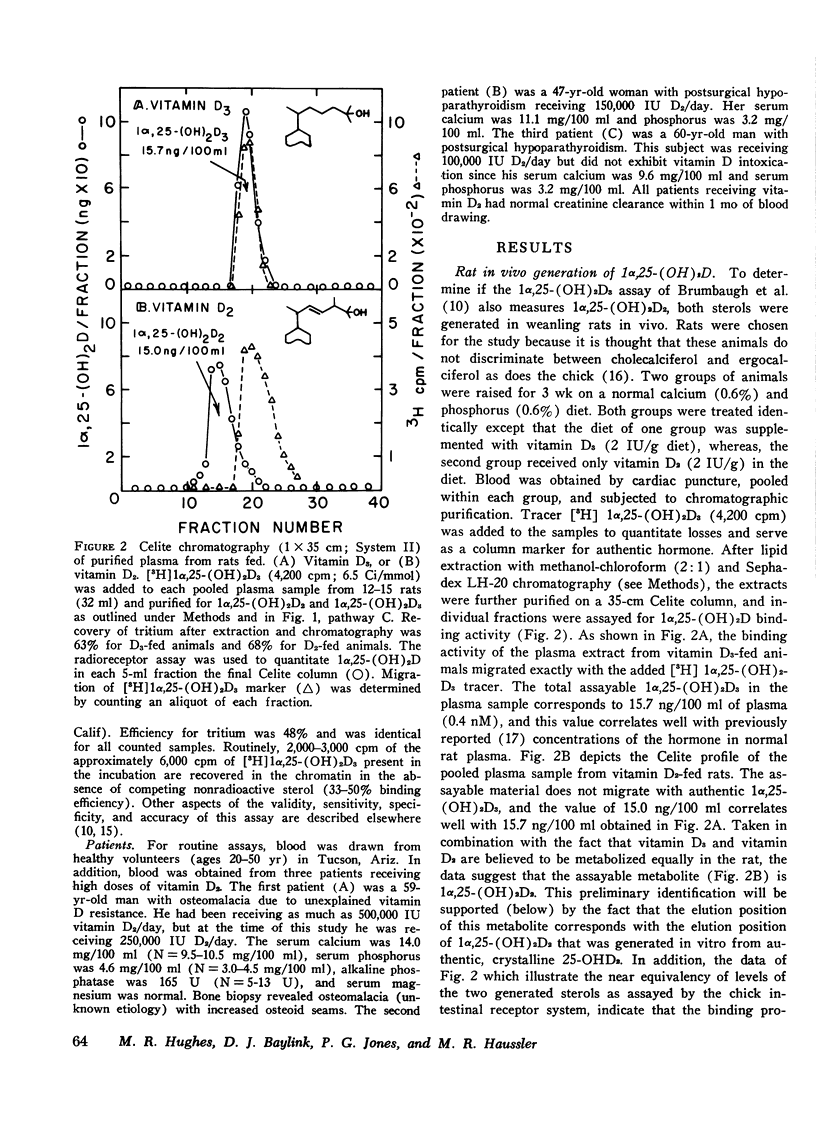
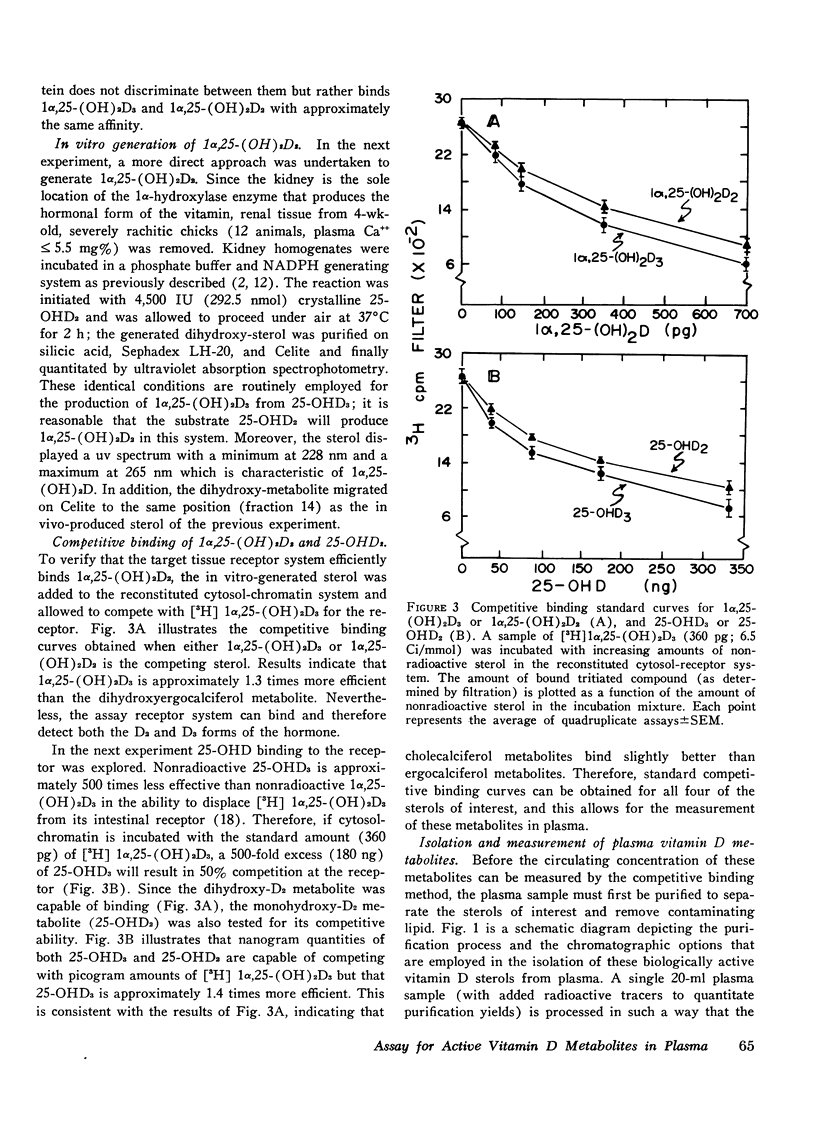
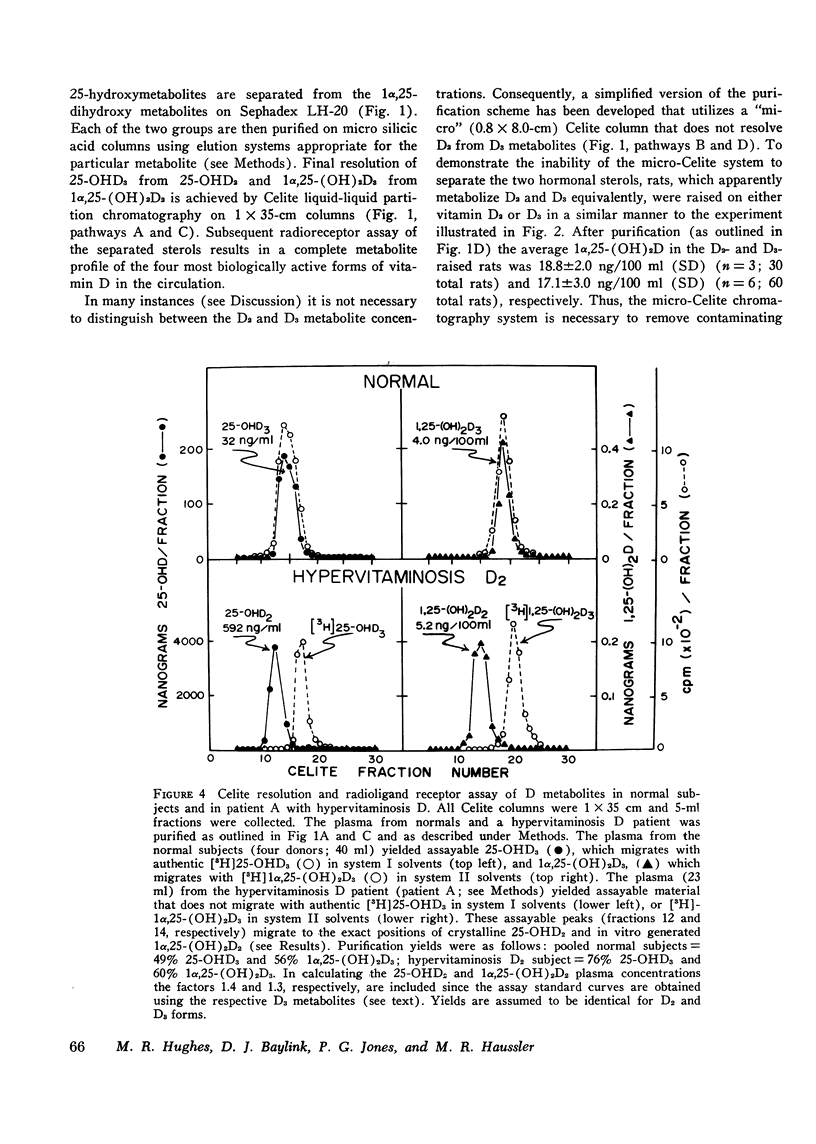
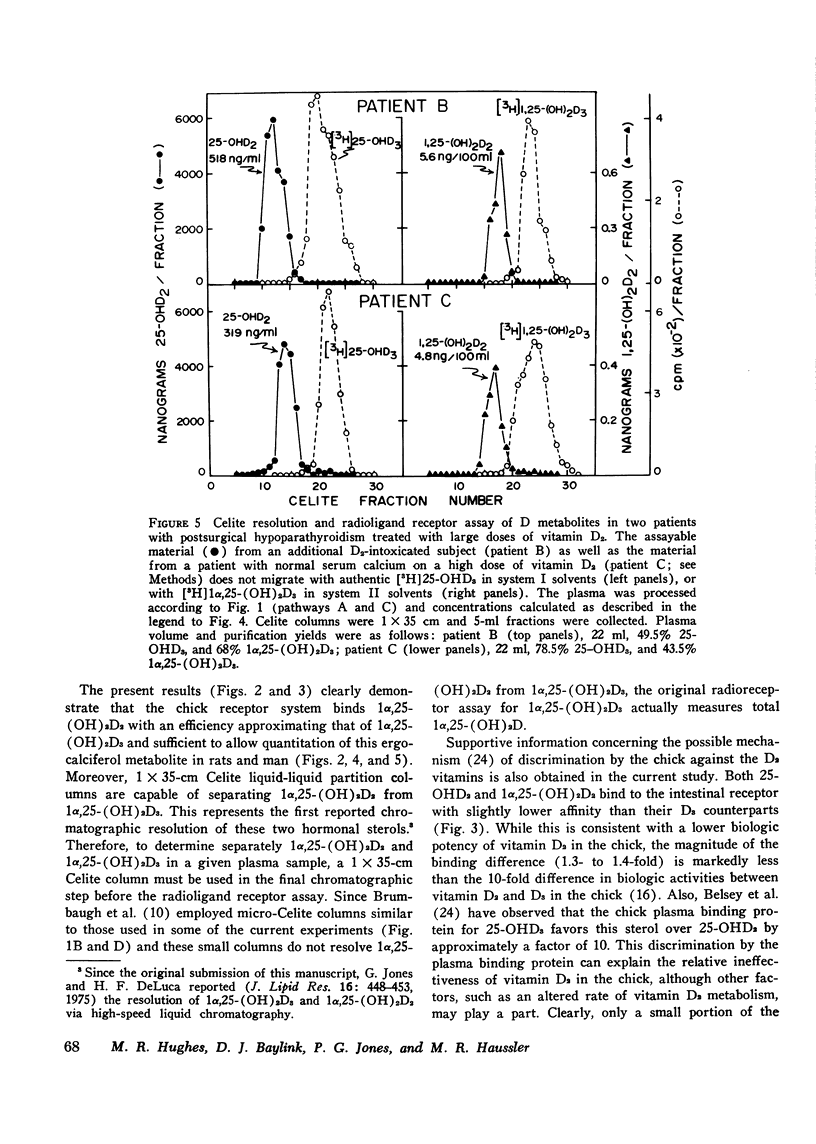
A competitive protein binding assay for measurement of the plasma concentration of 1 alpha, 25-dihydroxyvitamin D3 [1alpha, 25-(OH)2D3] has been extended to include the immediate precursor of this hormone, 25-hydroxyvitamin D3 (25-OHD3). In addition, the assay system is capable of measuring the two metabolic products of ergocalciferol, namely. 25-hydroxyvitamin D2 (25-OHD2) and 1alpha, 25-dihydroxyvitamin D2 [1alpha, 25-(OH)2D2]. The target tissue assay system consists of a high affinity cytosol receptor protein that binds the vitamin D metabolites and a limited number of acceptor sites on the nuclear chromatin. By utilizing a series of chromatographic purification steps, a single plasma sample can be assayed for any of the four vitamin D metabolites either individually or combined. Therefore, the assay procedure allows for both the quantitative and qualitative assessment of the total active vitamin D level in a given plasma sample. To show that the binding assay was capable of measuring 1alpha, 25-(OH)2D2 as well as 1alpha, 25 (OH)2D3, two groups of rats were raised. One group, supplemented with vitamin D3, produced assayable material that represented 1alpha, 25-(OH)2D3. The other group, fed only vitamin D2 in the diet, yielded plasma containing only 1alpha, 25-(OH)2D2 as the hormonal form of the vitamin. The circulating concentrations of the two active sterols were nearly identical (15 ng/100 ml) in both groups, indicating that the competitive binding assay can be used to measure both hormonal forms in plasma. In a separate experiment, 1alpha, 25-(OH)2D2 was generated in an in vitro kidney homogenate system using 25-OHD2 as substrate. Comparison of this sterol with 1alpha, 25-(OH)2D3 in the assay system showed very similar binding curves; the D2 form was slightly less efficient (77%). Comparison of the respective 25-hydroxy forms (25-OHD2 vs. 25-OHD3) at concentrations 500-fold that of 1alpha, 25-(OH)2D3, again suggested that the binding of the D2 metabolite was slightly less efficient (71%). Finally, the assay was employed to measure the total active vitamin D metabolite pools in the plasma of normal subjects and patients with varying degrees of hypervitaminosis D. The normal plasma levels of 25-OHD and 1alpha, 25-(OH)2D measured in Tucson adults were 25-40 ng/ml and 2.1-4.5 ng/100 ml, respectively. Both sterols were predominately (greater than 90%) in the form of vitamin D3 metabolites in this environment. Typical cases of hypervitaminosis D exhibited approximately a 15-fold increase in the plasma 25-OHD concentration, and a dramatic changeover to virtually all metabolites existing in the form of D2 vitamins. In contrast, the circulating concentration of 1alpha, 25-(OH)2D was not substantially enhanced in vitamin D-intoxicated patients. We therefore conclude that hypervitaminosis D is not a result of abnormal plasma levels of 1alpha, 25-(OH)2D but may be cuased by an excessive circulating concentration of 25-OHD.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsey R. E., DeLuca H. F., Potts J. T., Jr A rapid assay for 25-OH-vitamin D3 without preparative chromatography. J Clin Endocrinol Metab. 1974 Jun;38(6):1046–1051. doi: 10.1210/jcem-38-6-1046. [DOI] [PubMed] [Google Scholar]
- Belsey R. E., DeLuca H. F., Potts J. T., Jr Selective binding properties of vitamin D transport protein in chick plasma in vitro. Nature. 1974 Jan 25;247(5438):208–209. doi: 10.1038/247208a0. [DOI] [PubMed] [Google Scholar]
- Belsey R., Deluca H. F., Potts J. T., Jr Competitive binding assay for vitamin D and 25-OH vitamin D. J Clin Endocrinol Metab. 1971 Sep;33(3):554–557. doi: 10.1210/jcem-33-3-554. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler D. H., Bressler R., Haussler M. R. Radioreceptor assay for 1 alpha,25-dihydroxyvitamin D3. Science. 1974 Mar 15;183(4129):1089–1091. doi: 10.1126/science.183.4129.1089. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler D. H., Bursac K. M., Haussler M. R. Filter assay for 1alpha, 25-dihydroxyvitamin D3. Utilization of the hormone's target tissue chromatin receptor. Biochemistry. 1974 Sep 24;13(20):4091–4097. doi: 10.1021/bi00717a005. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974 Feb 25;249(4):1251–1257. [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1Alpha,25-dihydroxyvitamin D3 receptor: competitive binding of vitamin D analogs. Life Sci. 1973 Dec 16;13(12):1737–1746. doi: 10.1016/0024-3205(73)90120-3. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. Specific binding of 1alpha,25-dihydroxycholecalciferol to nuclear components of chick intestine. J Biol Chem. 1975 Feb 25;250(4):1588–1594. [PubMed] [Google Scholar]
- Counts S. J., Baylink D. J., Shen F. H., Sherrard D. J., Hickman R. O. Vitamin D intoxication in an anephric child. Ann Intern Med. 1975 Feb;82(2):196–200. doi: 10.7326/0003-4819-82-2-196. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D: the vitamin and the hormone. Fed Proc. 1974 Nov;33(11):2211–2219. [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Chyu K. J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971 Dec;33(6):992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Jr, Hahn T. J. Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature. 1973 Aug 24;244(5417):515–517. doi: 10.1038/244515a0. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Baylink D. J., Hughes M. R., Brumbaugh P. F., Wergedal J. E., Shen F. H., Nielsen R. L., Counts S. J., Bursac K. M., McCain T. A. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–165S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Boyce D. W., Littledike E. T., Rasmussen H. A rapidly acting metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1971 Jan;68(1):177–181. doi: 10.1073/pnas.68.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R. Characterization of the metabolites of vitamin D 3 in the chick. Steroids. 1972 Nov;20(5):639–650. doi: 10.1016/0039-128x(72)90021-9. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Rasmussen H. The metabolism of vitamin D 3 in the chick. J Biol Chem. 1972 Apr 25;247(8):2328–2335. [PubMed] [Google Scholar]
- Haussler M. R. Vitamin D: mode of action and biomedical applications. Nutr Rev. 1974 Sep;32(9):257–266. doi: 10.1111/j.1753-4887.1974.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Kleiner-Bossaller A., Schnoes H. K., Kasten P. M., Boyle I. T., DeLuca H. F. 1,24,25-Trihydroxyvitamin D3. A metabolite of vitamin D3 effective on intestine. J Biol Chem. 1973 Oct 10;248(19):6691–6696. [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Fraser D. R., Kodicek E., Morris H. R., Williams D. H. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971 Mar 26;230(5291):228–230. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- McNutt K. W., Haussler M. R. Nutritional effectiveness of 1,25-dihydroxycholecalciferol in preventing rickets in chicks. J Nutr. 1973 May;103(5):681–689. doi: 10.1093/jn/103.5.681. [DOI] [PubMed] [Google Scholar]
- Norman A. W. The mode of action of vitamin D. Biol Rev Camb Philos Soc. 1968 Feb;43(1):97–137. doi: 10.1111/j.1469-185x.1968.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Tucker G., 3rd, Gagnon R. E., Haussler M. R. Vitamin D 3 -25-hydroxylase: tissue occurrence and apparent lack of regulation. Arch Biochem Biophys. 1973 Mar;155(1):47–57. doi: 10.1016/s0003-9861(73)80008-6. [DOI] [PubMed] [Google Scholar]


