Abstract
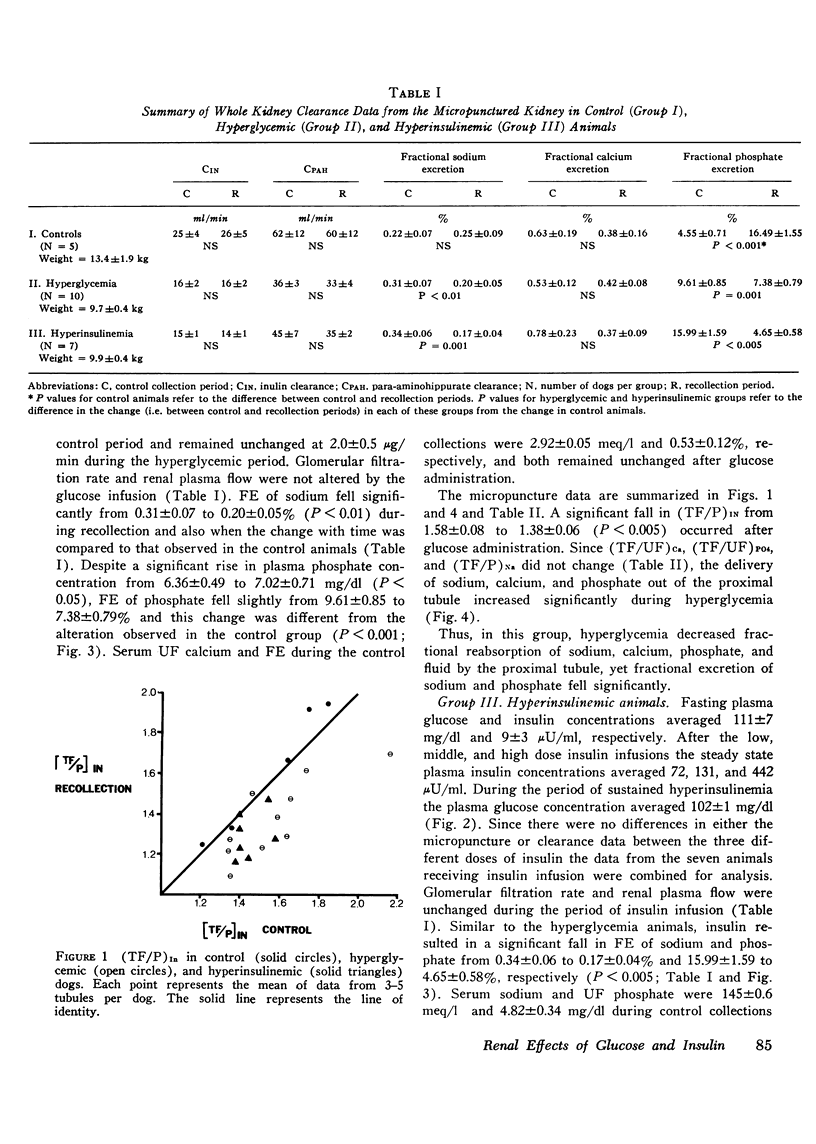
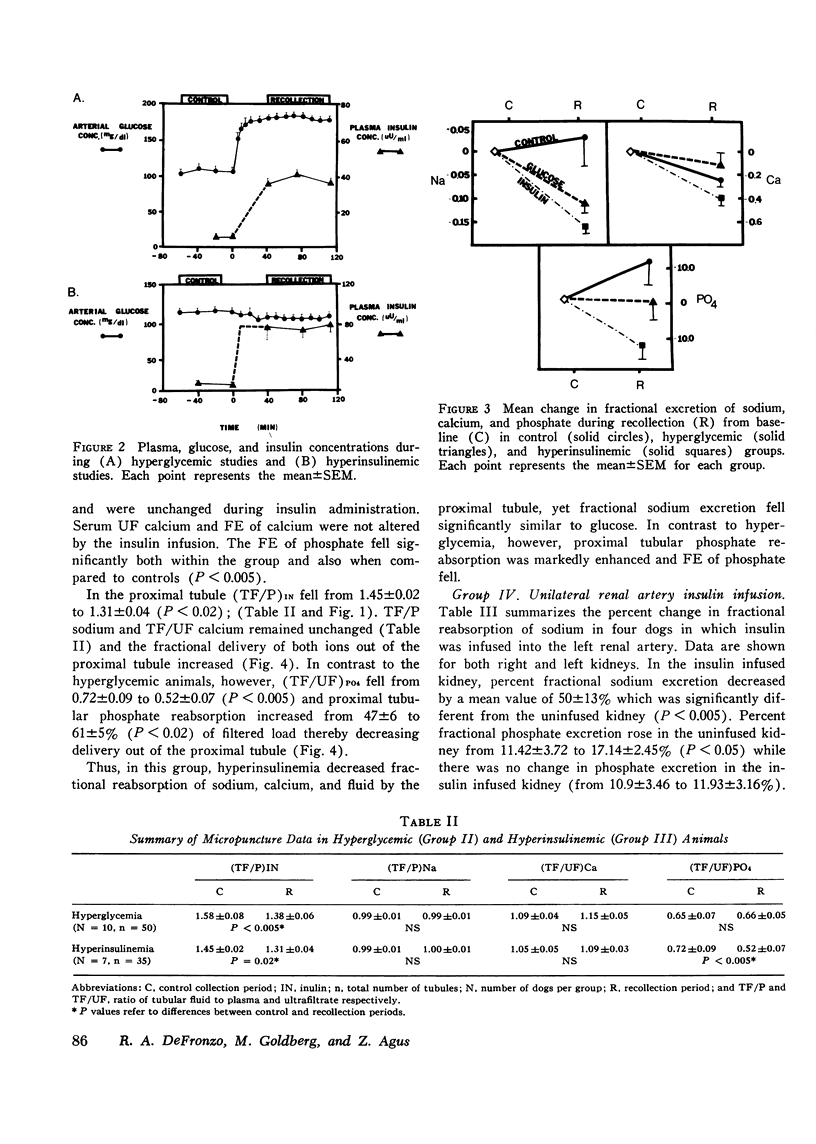
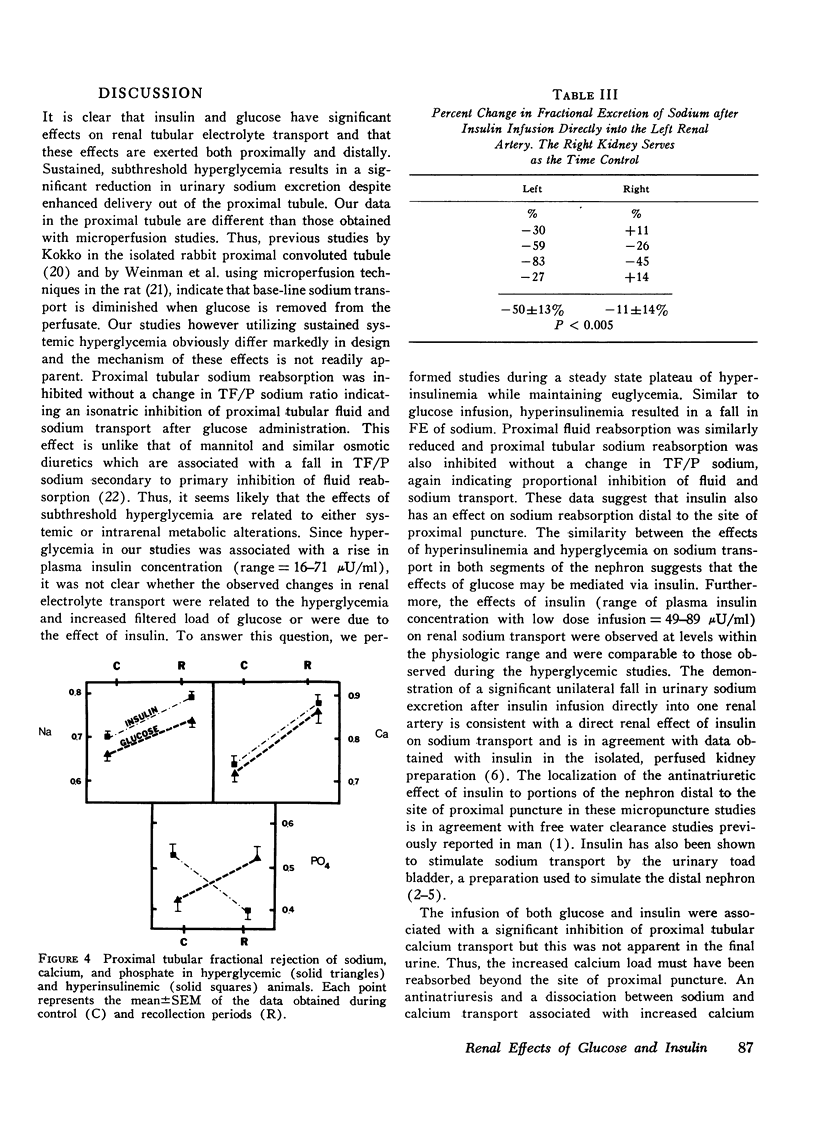
The effects of hyperglycemia and hyperinsulinemia on renal handling of sodium, calcium, and phosphate were studied in dogs employing the recollection micropuncture technique. Subthreshold sustained hyperglycemia resulted in an isonatric inhibition of proximal tubular sodium, fluid, calcium, and phosphate reabsorption by 8-14%. Fractional excretion of sodium and phosphate, however, fell (P is less than 0.01) indicating that the increased delivery of these ions was reabsorbed in portions of the nephron distal to the site of puncture and in addition net sodium and phosphate transport was enhanced resulting in a significant antinatriuresis and antiphosphaturia. The creation of a steady state plateau of hyperinsulinemia while maintaining the blood glucose concentration of euglycemic levels mimicked the effects of hyperglycemia on proximal tubular transport and fractional excretion of sodium and calcium. Tubular fluid to plasma insulin ratio fell, similar to the hyperglycemic studies. These results suggest that the effects of hyperglycemia on renal handling of sodium and calcium may be mediated through changes in plasma insulin concentration. In contrast to hyperglycemia, however, hyperinsulinemia cuased a significant fall in tubular fluid to plasma phosphate ratio with enhanced proximal tubular phosphate reabsorption (P is less than 0.02). This occurred concomitantly with a significant inhibition of proximal tubular sodium transport. These data indicate that insulin has a direct effect on proximal tubular phosphate reabsorption, and this effect of insulin is masked by the presence of increased amounts of unreabsorbed glucose in the tubule that ensues when hyperinsulinemia occurs secondary to hyperglycemia. Fractional excretion of phosphate fell significantly during insulin infusion but unlike the hyperglycemic studies, the fall in phosphate excretion could be entirely accounted for by enhanced proximal reabsorption.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Gardner L. B., Beck L. H., Goldberg M. Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am J Physiol. 1973 May;224(5):1143–1148. doi: 10.1152/ajplegacy.1973.224.5.1143. [DOI] [PubMed] [Google Scholar]
- Agus Z. S., Puschett J. B., Senesky D., Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3',5'-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971 Mar;50(3):617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel C., Kuntziger H., Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflugers Arch. 1970;317(2):93–109. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- BRUN C. A rapid method for the determination of para-aminohippuric acid in kidney function tests. J Lab Clin Med. 1951 Jun;37(6):955–958. [PubMed] [Google Scholar]
- COHEN J. J., BERGLUND F., LOTSPEICH W. D. Interrelations during renal tubular reabsorption in the dog among several anions showing a sensitivity to glucose and phlorizin. Am J Physiol. 1957 May;189(2):331–338. doi: 10.1152/ajplegacy.1957.189.2.331. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGLETON M. G., SHUSTER S. Glucose and phosphate excretion in the cat. J Physiol. 1954 Jun 28;124(3):613–622. doi: 10.1113/jphysiol.1954.sp005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M., THIER S., ROSENBERG L., SEGAL S. IMPAIRED RENAL TUBULAR FUNCTION INDUCED BY SUGAR INFUSION IN MAN. J Clin Endocrinol Metab. 1964 Dec;24:1318–1327. doi: 10.1210/jcem-24-12-1318. [DOI] [PubMed] [Google Scholar]
- François B., de Gasparo M., Crabbé J. Interaction between isolated amphibian skin and insulin. Arch Int Physiol Biochim. 1969 Aug;77(3):527–530. [PubMed] [Google Scholar]
- Garnett E. S., Nahmias C. The effect of glucose on the urinary excretion of sodium and hydrogen ion in man. Clin Sci Mol Med. 1974 Dec;47(6):589–598. doi: 10.1042/cs0470589. [DOI] [PubMed] [Google Scholar]
- Ginsburg J. M. Effect of glucose and free fatty acid on phosphate transport in dog kidney. Am J Physiol. 1972 May;222(5):1153–1160. doi: 10.1152/ajplegacy.1972.222.5.1153. [DOI] [PubMed] [Google Scholar]
- HERRERA F. C., WHITTEMBURY G., PLANCHART A. Effect of insulin on short-circuit current across isolated frog skin in the presence of calcium and magnesium. Biochim Biophys Acta. 1963 Jan 15;66:170–172. doi: 10.1016/0006-3002(63)91182-x. [DOI] [PubMed] [Google Scholar]
- HODGKINSON A., HEATON F. W. THE EFFECT OF FOOD INGESTION ON THE URINARY EXCRETION OF CALCIUM AND MAGNESIUM. Clin Chim Acta. 1965 Apr;11:354–362. doi: 10.1016/0009-8981(65)90226-3. [DOI] [PubMed] [Google Scholar]
- HUFFMAN E. R., HLAD C. J., Jr, WHIPPLE N. E., ELRICK H. The influence of blood glucose on the renal clearance of phosphate. J Clin Invest. 1958 Mar;37(3):369–379. doi: 10.1172/JCI103616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter H. R., Mercado A., Rutherford W. E., Rodriguez H., Slatopolsky E., Klahr S. Effects of phosphate depletion and parathyroid hormone on renal glucose reabsorption. Am J Physiol. 1974 Dec;227(6):1422–1427. doi: 10.1152/ajplegacy.1974.227.6.1422. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO 3 , and amino acids. J Clin Invest. 1973 Jun;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITAN B. A. Effect in normal man of hyperglycemia and glycosuria on excretion and reabsorption of phosphate. J Appl Physiol. 1951 Sep;4(3):224–226. doi: 10.1152/jappl.1951.4.3.224. [DOI] [PubMed] [Google Scholar]
- Lennon E. J., Lemann J., Jr, Piering W. F., Larson L. S. The effect of glucose on urinary cation excretion during chronic extracellular volume expansion in normal man. J Clin Invest. 1974 May;53(5):1424–1433. doi: 10.1172/JCI107690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon E. J., Piering W. F. A comparison of the effects of glucose ingestion and NH4Cl acidosis on urinary calcium and magnesium excretion in man. J Clin Invest. 1970 Jul;49(7):1458–1465. doi: 10.1172/JCI106363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman R. D., Adler S., Yiengst M. J., Beard E. S. Influence of various nutrients on urinary divalent cation excretion. J Lab Clin Med. 1967 Aug;70(2):236–245. [PubMed] [Google Scholar]
- Lindeman R. D., Adler S., Yiengst M. J., Beard E. S. Natriuresis and carbohydrate-induced antinatriuresis after overnight fast and hydration. Nephron. 1970 Jul;7(4):289–300. doi: 10.1159/000179830. [DOI] [PubMed] [Google Scholar]
- Nizet A., Lefebvre P., Crabbé J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 1971;323(1):11–20. doi: 10.1007/BF00586561. [DOI] [PubMed] [Google Scholar]
- Seely J. F., Dirks J. H. Micropuncture study of hypertonic mannitol diuresis in the proximal and distal tubule of the dog kidney. J Clin Invest. 1969 Dec;48(12):2330–2340. doi: 10.1172/JCI106199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. H., Motto G. S., Kukreja S. C., Hargis G. K., Williams G. A. Stimulation of the secretion of parathyroid hormone during hypoglycemic stress. J Clin Endocrinol Metab. 1975 Oct;41(4):692–696. doi: 10.1210/jcem-41-4-692. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhey S. S., Allan F. N. The Relationship of Phosphates to Carbohydrate Metabolism: Time Relationship of the Changes in Phosphate Excretion caused by Insulin and Sugar. Biochem J. 1924;18(5):1170–1184. doi: 10.1042/bj0181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staum B. B., Hamburger R. J., Goldberg M. Tracer microinjection study of renal tubular phosphate reabsorption in the rat. J Clin Invest. 1972 Sep;51(9):2271–2276. doi: 10.1172/JCI107036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S. F. Micropuncture studies of phosphate transport in the proximal tubule of the dog. The relationship to sodium reabsorption. J Clin Invest. 1974 Jan;53(1):143–153. doi: 10.1172/JCI107532. [DOI] [PMC free article] [PubMed] [Google Scholar]


