Abstract
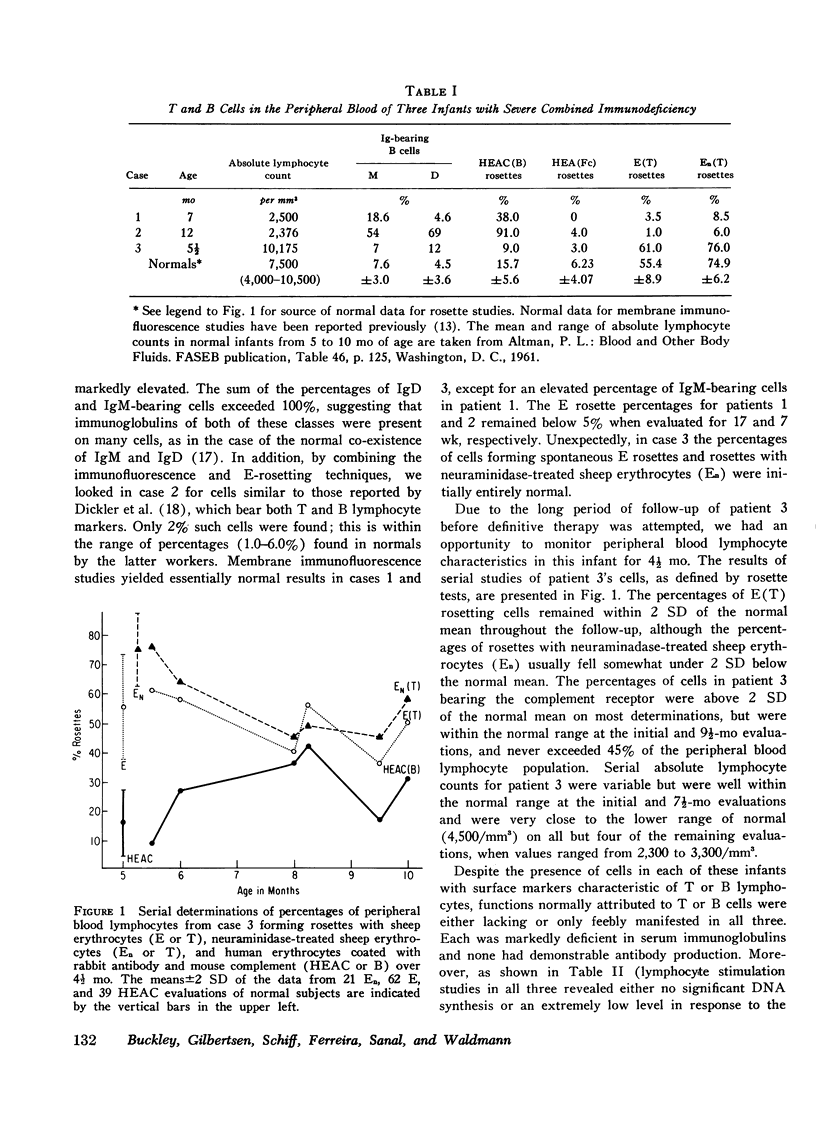
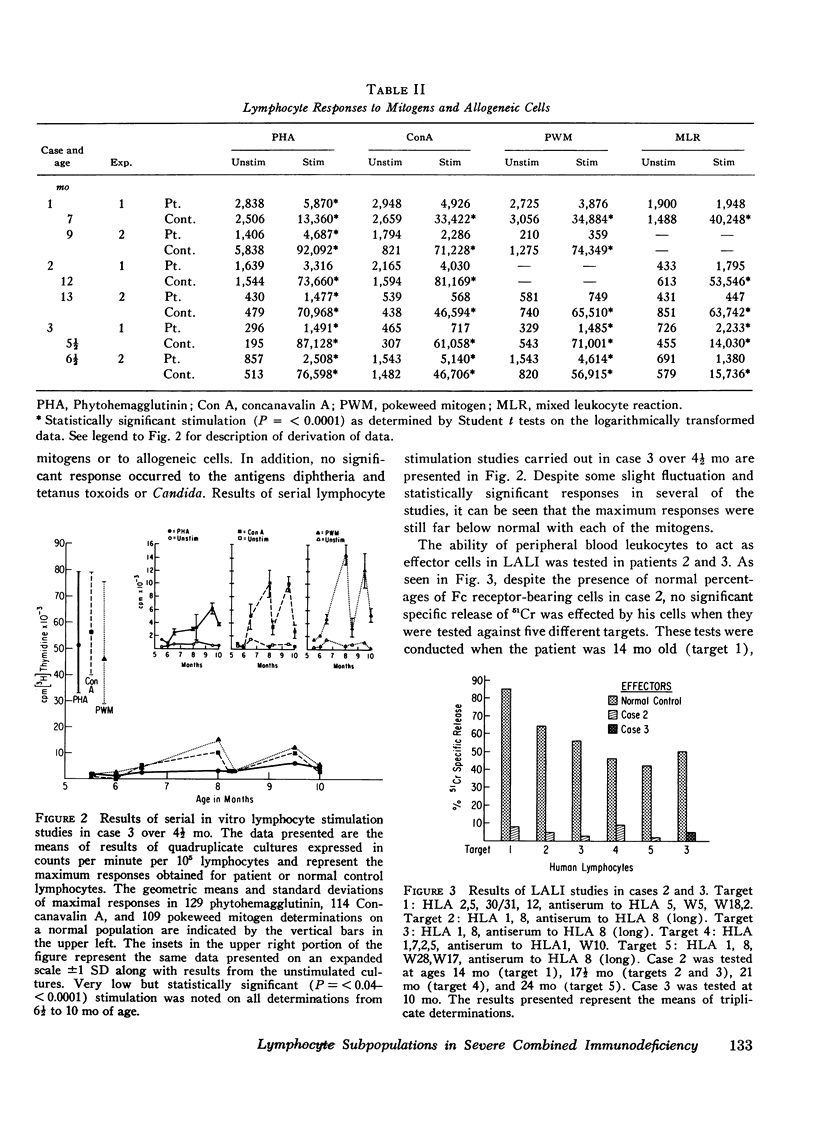
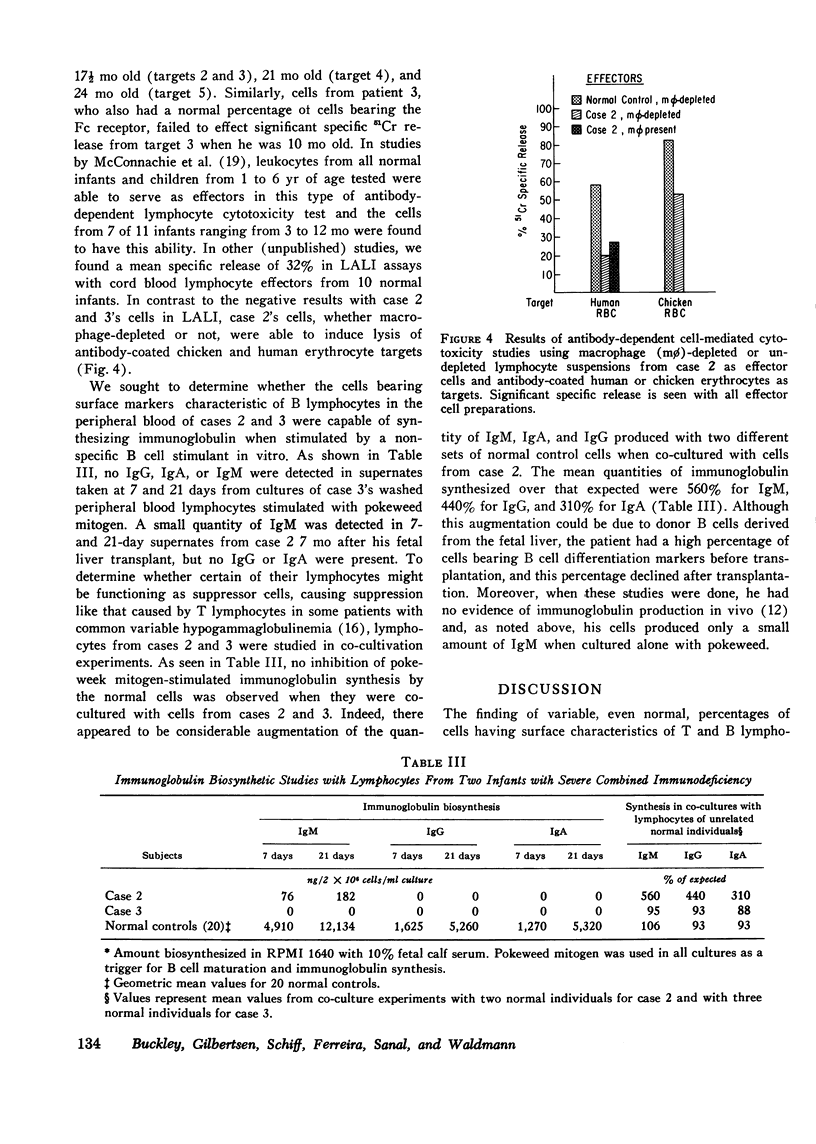
Surface markers typical of T and B lymphocytes were present on varying proportions of peripheral blood lymphocytes from three infants with severe combined immunodeficiency disease. Despite this, functions mediated by T and B cells were either absent or very minimal in all three, including cell-mediated responses in vivo; the in vitro proliferative response to mitogens, allogeneic cells, or antigens; effector cell function in lymphocyte-antibody lymphocytolytic interaction assays; and in vitro synthesis of IgG, IgA, and IgM. In contrast, mononuclear cells from one of the infants were tested and found capable of lysing both human and chicken antibody-coated erythrocyte targets normally. Co-cultivation experiments with unrelated normal control lymphocytes failed to demonstrate suppressor cell activity for immunoglobulin synthesis in these infants. Augmentations of immunoglobulin production from 310 to 560% over that expected on the basis of individual culture data were noted in co-cultures of one of the infants' cells with two different unrelated normal control cells. These findings suggest that that infant may have had a T helper cell defect or that his T cells were unable to produce soluble factors necessary for B cell differentiation. The finding of cells with differentiation markers characteristic of T and B lymphocytes in each of these patients, though in variable quantities, is further evidence for heterogeneity among patients with the clinical syndrome of severe combined immunodeficiency and argues against the concept that their immunodeficiency was due to a stem cell defect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley R. H., Whisnant K. J., Schiff R. I., Gilbertsen R. B., Huang A. T., Platt M. S. Correction of severe combined immunodeficiency by fetal liver cells. N Engl J Med. 1976 May 13;294(20):1076–1081. doi: 10.1056/NEJM197605132942002. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Keightley R. G., Wu L. Y., Lawton A. R., 3rd Developmental defects of T and B cell lines in humans. Transplant Rev. 1973;16:51–84. doi: 10.1111/j.1600-065x.1973.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Adkinson N. F., Jr, Terry W. D. Evidence for individual human peripheral blood lymphocytes bearing both B and T cell markers. Nature. 1974 Jan 25;247(5438):213–215. doi: 10.1038/247213a0. [DOI] [PubMed] [Google Scholar]
- Froland S. S., Wisloff F., Michaelsen T. E. Human lymphocytes with receptors for IgG. A population of cells distinct from T- and B-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):124–138. doi: 10.1159/000231207. [DOI] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Park B. H., Biggar W. D., Good R. A. B and T lymphocytes in primary immunodeficiency disease in man. J Clin Invest. 1973 Apr;52(4):919–928. doi: 10.1172/JCI107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Schneeberger E., Gatien J., Rosen F. S., Merler E. Synthesis of an M component by circulating B lymphocytes in severe combined immunodeficiency. N Engl J Med. 1974 Mar 28;290(13):726–728. doi: 10.1056/NEJM197403282901308. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Luckasen J. R., Sabad A., Gajl-Peczalska K. J., Kersey J. H. Lymphocytes bearing complement receptors, surface immunoglobulins and sheep erythrocyte receptors in primary immunodeficiency diseases. Clin Exp Immunol. 1974 Apr;16(4):535–540. [PMC free article] [PubMed] [Google Scholar]
- McConnachie P. R., Rachelefsky G., Stiehm E. R., Terasaki P. I. Antibody-dependent lymphocyte killer function and age. Pediatrics. 1973 Dec;52(6):795–800. [PubMed] [Google Scholar]
- Meuwissen H. J., Bach F. H., Hong R., Good R. A. Lymphocyte studies in congenital thymic dysplasia: The one-way stimulation test. J Pediatr. 1968 Feb;72(2):177–185. doi: 10.1016/s0022-3476(68)80306-3. [DOI] [PubMed] [Google Scholar]
- Meuwissen H. J., Pollara B., Pickering R. J. Combined immunodeficiency disease associated with adenosine deaminase deficiency. Report on a workshop held in Albany, New York, October 1, 1973. J Pediatr. 1975 Feb;86(2):169–181. doi: 10.1016/s0022-3476(75)80463-x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N., Richie E., Montgomery J. R., Wilson R., Fernbach D. J. Letter: T and B cell characteristics in combined immunodeficiency. N Engl J Med. 1974 Sep 26;291(13):678–678. doi: 10.1056/NEJM197409262911313. [DOI] [PubMed] [Google Scholar]
- Polmar S. H., Wetzler E. M., Stern R. C., Hirschhorn R. Restoration of in-vitro lymphocyte responses with exogenous adenosine deaminase in a patient with severe combined immunodeficiency. Lancet. 1975 Oct 18;2(7938):743–746. doi: 10.1016/s0140-6736(75)90726-6. [DOI] [PubMed] [Google Scholar]
- Pyke K. W., Dosch H., Ipp M. M., Gelfand E. W. Demonstration of an intrathymic defect in a case of severe combined immunodeficiency disease. N Engl J Med. 1975 Aug 28;293(9):424–428. doi: 10.1056/NEJM197508282930904. [DOI] [PubMed] [Google Scholar]
- Rachelefsky G. S., McConnachie P. R., Ammann A. J., Terasaki P. I., Stiehm E. R. Antibody-dependent lymphocyte killer function in human immunodeficiency diseases. Clin Exp Immunol. 1975 Jan;19(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. I., Buckley R. H., Gilbertsen R. B., Metzgar R. S. Membrane receptors and in vitro responsiveness of lymphocytes in human immunodeficiency. J Immunol. 1974 Jan;112(1):376–386. [PubMed] [Google Scholar]
- Trinchieri G., De Marchi M., Mayr W., Savi M., Ceppellini R. Lymphocyte antibody lymphocytolytic interaction (LALI) with special emphasis on HL-A. Transplant Proc. 1973 Dec;5(4):1631–1649. [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Wisloff F., Froland S. S., Michaelsen T. E. Antibody-dependent cytotoxicity mediated by human Fc-receptor-bearing cells lacking markers for B- and T-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):139–154. doi: 10.1159/000231208. [DOI] [PubMed] [Google Scholar]


