Abstract
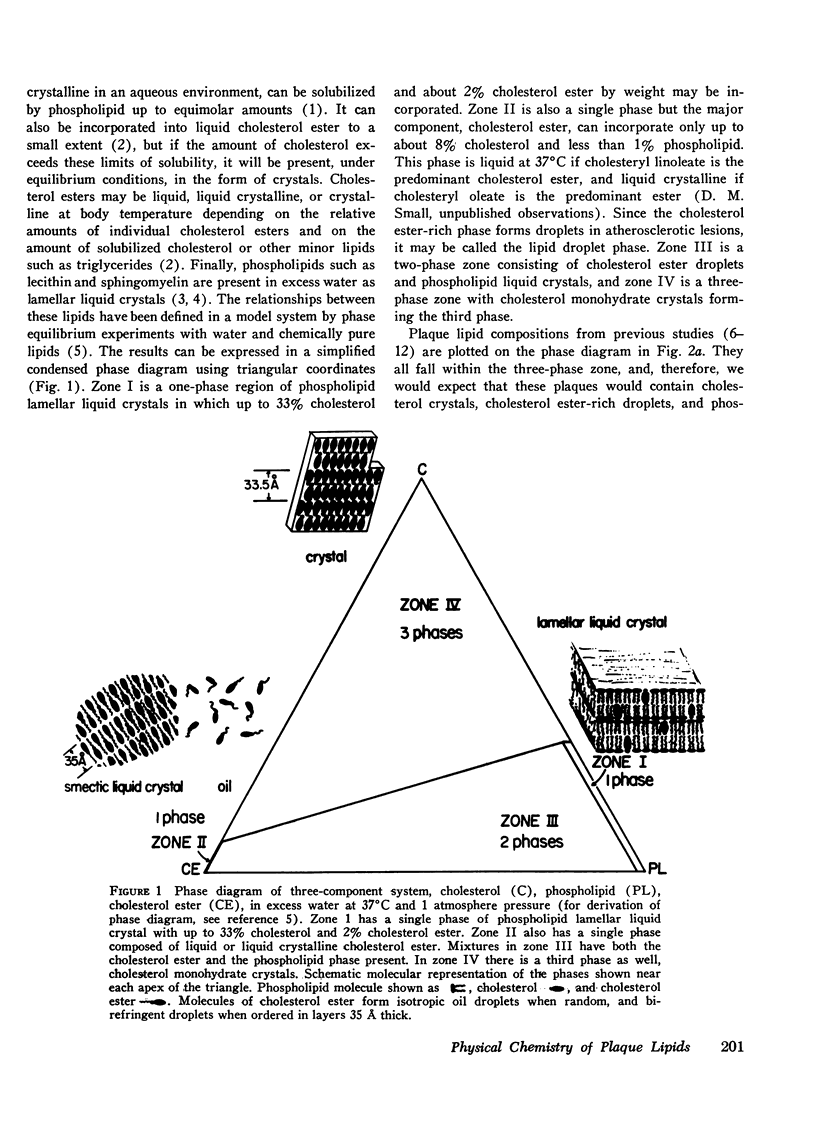
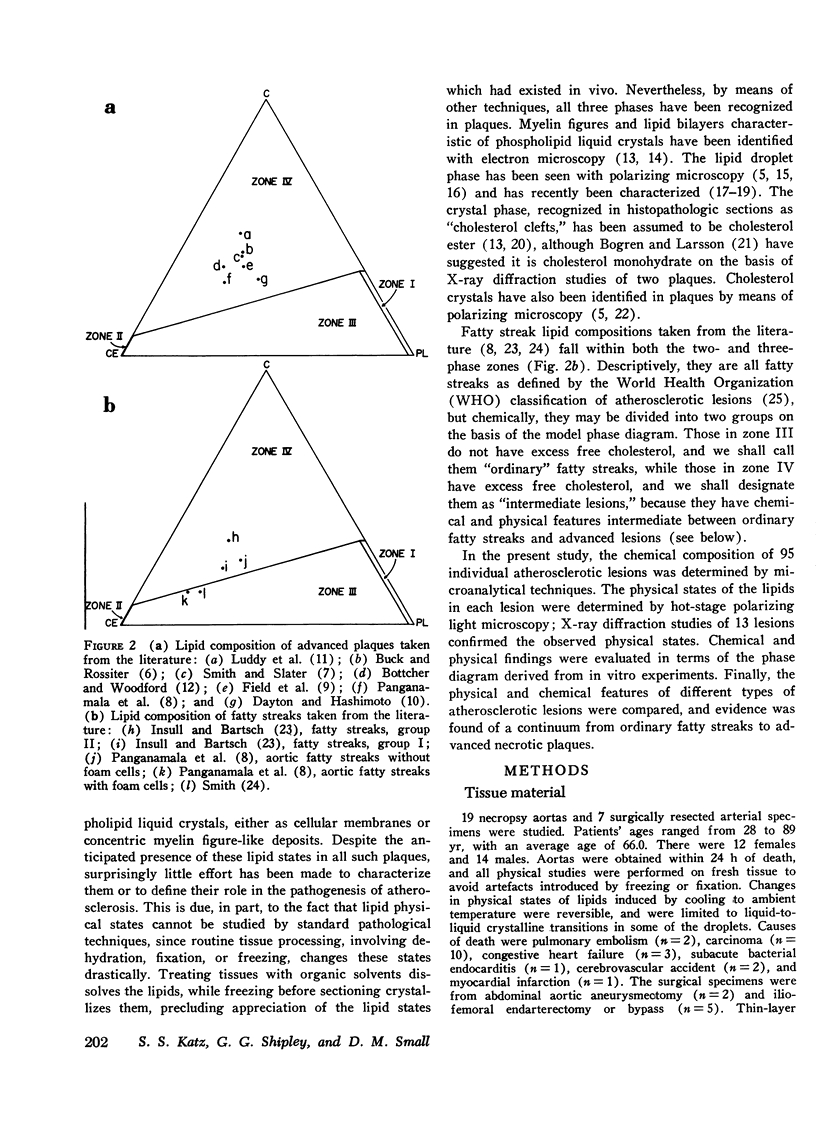
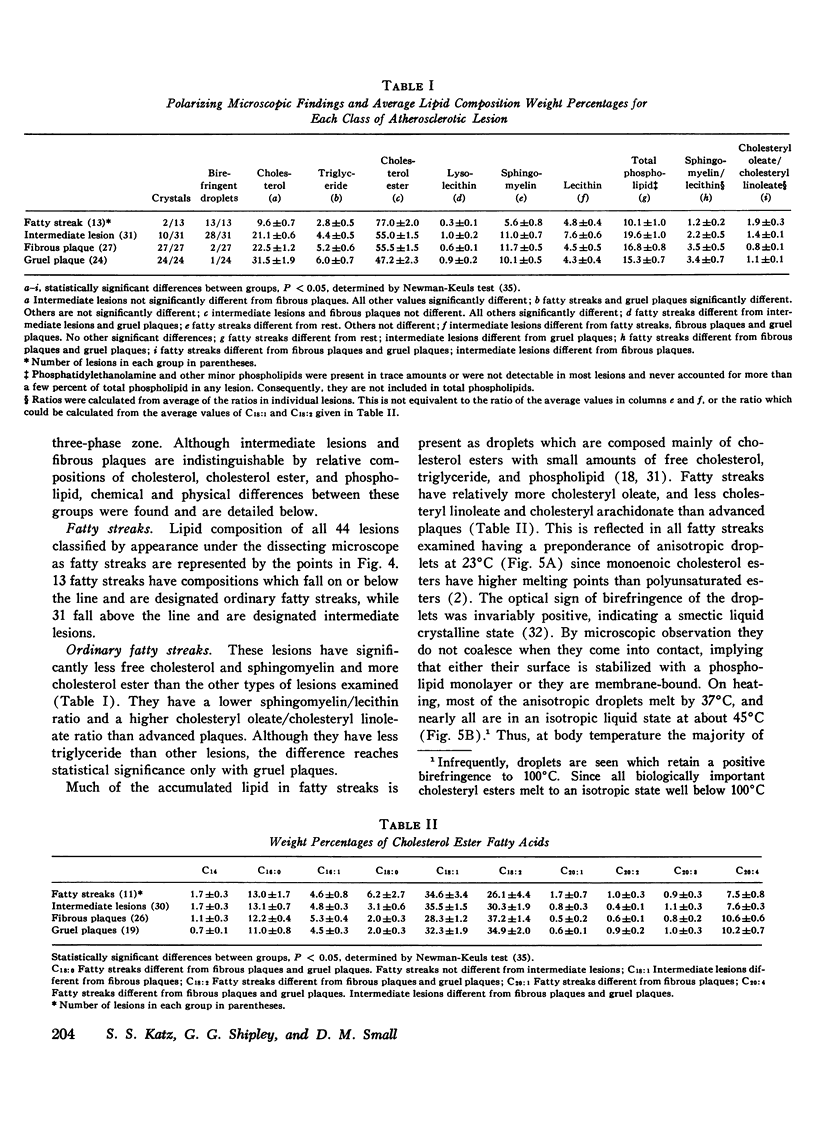
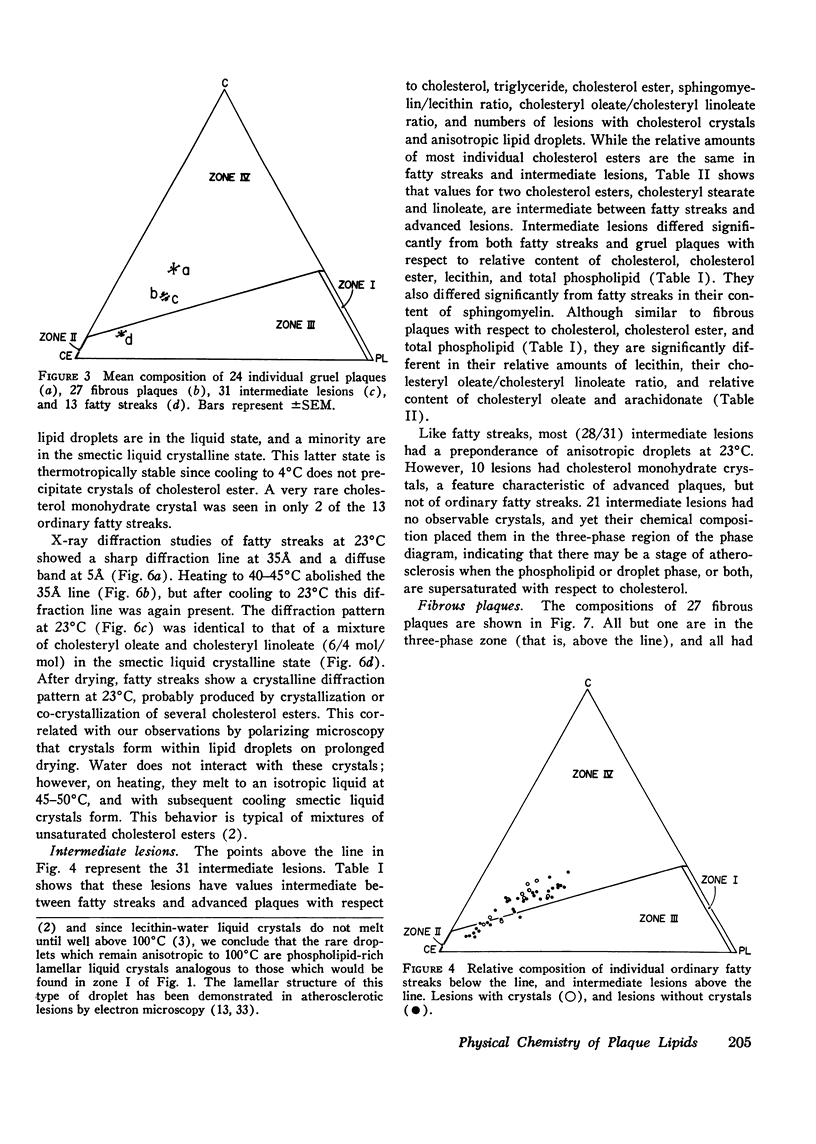
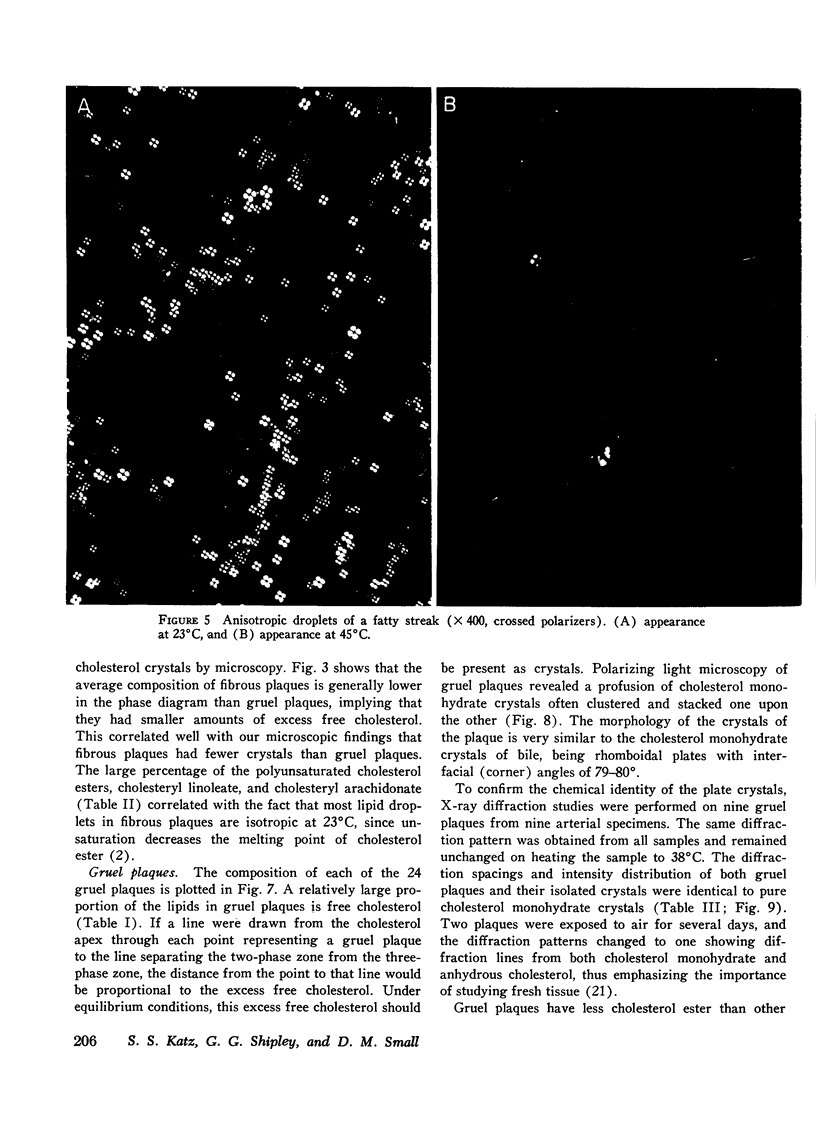
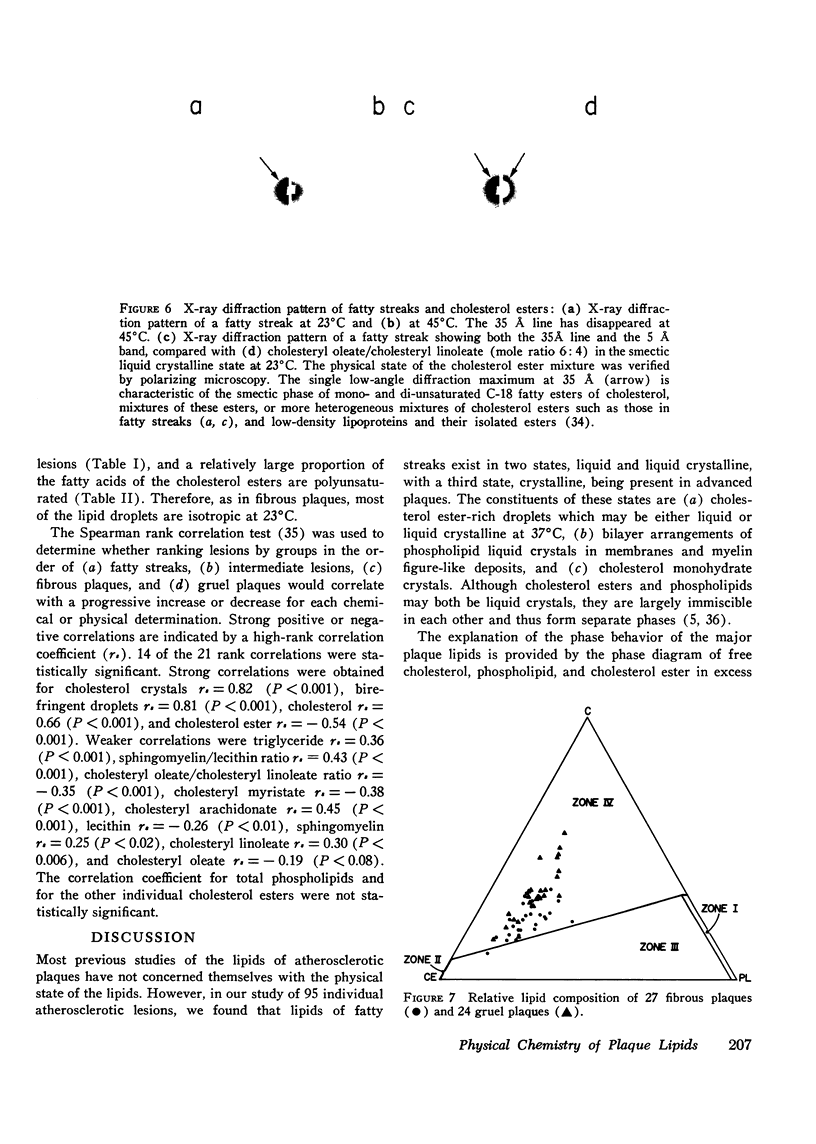
95 individual human atherosclerotic lesions from 26 persons were classified into three groups under the dissecting microscope: fatty streaks, fibrous plaques, and gruel (atheromatous) plaques. Each lesion was isolated by microdissection, its lipid composition determined by chromatography, and the physical states of the lipids identified by polarizing microscopy and in some cases by X-ray diffraction. The composition of each lesion was plotted on the in vitro phase diagram of the major lipids of plaques: cholesterol, cholesterol ester, and phospholipid. The observed physical states were compared with those predicted by the location of the lipid composition on the phase diagram. The most severe lesions (gruel plaques) had an average lipid composition of cholesterol 31.5+/-1.9%, cholesterol ester 47.2+/-2.3%, and phospholipid 15.3+/-0.5%. Their compositions fell within the three-phase zone of the phase diagram, predicting the lipids to be separated into a cholesterol crystal phase, a cholesterol ester oily phase and a phospholipid liquid crystalline phase. In addition to the phospholipid liquid crystalline phase of membranes and myelin-like figures demonstrable by electron microscopy, polarizing microscopy revealed the other two predicted phases, isotropic cholesterol ester-rich droplets and cholesterol crystals. X-ray diffraction studies verified the identity of the crystals as cholesterol monohydrate. Fibrous plaques also had an average lipid composition within the three-phase zone of the phase diagram. Polarizing microscopy revealed the presence of cholesterol monohydrate crystals and lipid droplets in all of these lesions; the droplets were predominately isotropic in 28 of the 31 fibrous plaques. Although these lesions had less free cholesterol and more cholesterol ester than gruel plaques, they were otherwise similar. Fatty streaks had compositions within both the two- and three-phase zones of the phase diagram. Compared with gruel plaques, the fatty streaks within the two-phase zone, defined as "ordinary," had more cholesterol ester, less free cholesterol, a higher cholesteryl oleate/cholesteryl linoleate ratio, a lower sphingomyelin/lecithin ratio, more anisotropic lipid droplets, and rare or no cholesterol crystals. Those lesions within the three-phase zone had many chemical and physical features intermediate between ordinary fatty streaks and gruel plaques. Moreover, 68% of these "intermediate" lesions had no cholesterol crystals by polarizing microscopy in spite of their compositions being within the three-phase zone, indicating the cholesterol ester oily phase or the phospholipid phase or both were supersaturated with cholesterol. Identification of this group of intermediate lesions provides evidence that some fatty streaks may be precursors of advanced plaques.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGREN H., LARSSON K. AN X-RAY-DIFFRACTION STUDY OF CRYSTALLINE CHOLESTEROL IN SOME PATHOLOGICAL DEPOSITS IN MAN. Biochim Biophys Acta. 1963 Jul 23;75:65–69. doi: 10.1016/0006-3002(63)90580-8. [DOI] [PubMed] [Google Scholar]
- BOTTCHER C. J., WOODFORD F. P. Chemical changes in the arterial wall associated with atherosclerosis. Fed Proc. 1962 Jul-Aug;21(4):15–19. [PubMed] [Google Scholar]
- BUCK R. C., ROSSITER R. J. Lipids of normal and atherosclerotic aortas; a chemical study. AMA Arch Pathol. 1951 Feb;51(2):224–237. [PubMed] [Google Scholar]
- CARROLL K. K. Quantitative estimation of peak areas in gas-liquid chromatography. Nature. 1961 Jul 22;191:377–378. doi: 10.1038/191377a0. [DOI] [PubMed] [Google Scholar]
- CHOBANIAN A. V., HOLLANDER W. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J Clin Invest. 1962 Sep;41:1732–1737. doi: 10.1172/JCI104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton S., Hashimoto S., Pearce M. L. Influence of a diet high in unsaturated fat upon composition of arterial tissue and atheromata in man. Circulation. 1965 Dec;32(6):911–924. doi: 10.1161/01.cir.32.6.911. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M., Lees R. S., George P. K. Thermal transitions in human plasma low density lipoproteins. Science. 1975 Oct 24;190(4212):392–394. doi: 10.1126/science.170681. [DOI] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- FIELD H., Jr, SWELL L., SCHOOLS P. E., Jr, TREADWELL C. R. Dynamic aspects of cholesterol metabolism in different areas of the aorta and other tissues in man and their relationship to atherosclerosis. Circulation. 1960 Oct;22:547–558. doi: 10.1161/01.cir.22.4.547. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ghidoni J. J., O'Neal R. M. Recent advances in molecular pathology: a review ultrastructure of human atheroma. Exp Mol Pathol. 1967 Dec;7(3):378–400. doi: 10.1016/0014-4800(67)90049-4. [DOI] [PubMed] [Google Scholar]
- Hata Y., Hower J., Insull W., Jr Cholesteryl ester-rich inclusions from human aortic fatty streak and fibrous plaque lesions of atherosclerosis. I. Crystalline properties, size and internal structure. Am J Pathol. 1974 Jun;75(3):423–456. [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Insull W., Jr Significance of cholesterol esters as liquid crystal in human atherosclerosis. Jpn Circ J. 1973 Mar;37(3):269–275. doi: 10.1253/jcj.37.269. [DOI] [PubMed] [Google Scholar]
- Insull W., Jr, Bartsch G. E. Cholesterol, triglyceride, and phospholipid content of intima, media, and atherosclerotic fatty streak in human thoracic aorta. J Clin Invest. 1966 Apr;45(4):513–523. doi: 10.1172/JCI105365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan S. N., Connor W. E., Baker W. H., Bhattacharyya A. K. The turnover of cholesterol in human atherosclerotic arteries. J Clin Invest. 1974 Aug;54(2):366–377. doi: 10.1172/JCI107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak M. J., Loomis C. R., Shipley G. G., Small D. M. The ternary phase diagram of lecithin, cholesteryl linolenate and water: phase behavior and structure. J Mol Biol. 1974 Jun 25;86(2):325–339. doi: 10.1016/0022-2836(74)90022-9. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Iannaccone P. M. Atheromatous emboli in renal biopsies. An ultrastructural study. Am J Pathol. 1975 Feb;78(2):261–276. [PMC free article] [PubMed] [Google Scholar]
- LUDDY F. E., BARFORD R. A., RIEMENSCHNEIDER R. W. Fatty acid composition of component lipides from human plasma and atheromas. J Biol Chem. 1958 Jun;232(2):843–851. [PubMed] [Google Scholar]
- Lang P. D., Insull W., Jr Lipid droplets in atherosclerotic fatty streaks of human aorta. J Clin Invest. 1970 Aug;49(8):1479–1488. doi: 10.1172/JCI106365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganamala R. V., Geer J. C., Sharma H. M., Cornwell D. G. The gross and histologic appearance and the lipid composition of normal intima and lesions from human coronary arteries and aorta. Atherosclerosis. 1974 Jul-Aug;20(1):93–104. doi: 10.1016/0021-9150(74)90083-5. [DOI] [PubMed] [Google Scholar]
- STEWART G. T. Liquid crystals of lipid in normal and atheromatous tissue. Nature. 1959 Mar 28;183(4665):873–875. doi: 10.1038/183873a0. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Avecilla L. S., Small D. M. Phase behavior and structure of aqueous dispersions of sphingomyelin. J Lipid Res. 1974 Mar;15(2):124–131. [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Smith E. B., Slater R. S. The microdissection of large atherosclerotic plaques to give morphologically and topographically defined fractions for analysis. 1. The lipids in the isolated fractions. Atherosclerosis. 1972 Jan-Feb;15(1):37–56. doi: 10.1016/0021-9150(72)90036-6. [DOI] [PubMed] [Google Scholar]
- Weller R. O., Clark R. A., Oswald W. B. Stages in the formation and metabolism in intracellular lipid droplets in atherosclerosis. An electron microscopical and biochemical study. J Atheroscler Res. 1968 Mar-Apr;8(2):249–263. doi: 10.1016/s0368-1319(68)80061-4. [DOI] [PubMed] [Google Scholar]