Abstract
Many patients with diabetes mellitus (both type 1 and type 2) require therapy to maintain normal fasting glucose levels. To develop a novel treatment for these individuals, we used phage display technology to target the insulin receptor (INSR) complexed with insulin and identified a high affinity, allosteric, human monoclonal antibody, XMetA, which mimicked the glucoregulatory, but not the mitogenic, actions of insulin. Biophysical studies with cultured cells expressing human INSR demonstrated that XMetA acted allosterically and did not compete with insulin for binding to its receptor. XMetA was found to function as a specific partial agonist of INSR, eliciting tyrosine phosphorylation of INSR but not the IGF-IR. Although this antibody activated metabolic signaling, leading to enhanced glucose uptake, it neither activated Erk nor induced proliferation of cancer cells. In an insulin resistant, insulinopenic model of diabetes, XMetA markedly reduced elevated fasting blood glucose and normalized glucose tolerance. After 6 weeks, significant improvements in HbA1c, dyslipidemia, and other manifestations of diabetes were observed. It is noteworthy that hypoglycemia and weight gain were not observed during these studies. These studies indicate, therefore, that allosteric monoclonal antibodies have the potential to be novel, ultra-long acting, agents for the regulation of hyperglycemia in diabetes.
Absolute or relative insulinopenia are features of diabetes. Type 1 diabetes (T1DM) occurs in ∼10% of patients with diabetes and is associated with absolute insulinopenia (1). In most individuals who develop type 2 diabetes (T2DM), both insulin resistance and relative insulinopenia are present (2–12). It has been estimated that β-cell function has declined by 80% at the time of the initial diagnosis of T2DM (13). In late T2DM, as in T1DM, many patients are administered a long-acting insulin to control fasting blood glucose.
Insulin acts by binding to the insulin receptor (INSR) on the cell surface, a process that activates cell signaling (14). When activated, the INSR undergoes autophosphorylation, followed by the recruitment of insulin receptor signaling molecules, including the IRS proteins and members of the phosphotidylinositol 3-kinase (PI3K)/Akt pathway (15). As a result, there is translocation of glucose transporters, including GLUT4, to the cell surface (16). These processes are impeded in the insulin resistant state of T2DM and further compromised under conditions of insufficient insulin (17). Activation of INSR also stimulates Erk phosphorylation, a mitogenic signal postulated to contribute to inflammation, cancer cell proliferation, and deleterious cardiovascular outcomes (18).
Long-acting, or basal, insulins, such as insulin detemir and insulin glargine, are insulin analogs that are now used therapeutically in patients with diabetes (19). Although these agents are effective at lowering fasting blood glucose, they must be administered subcutaneously, once or twice daily. As insulin analogs, they carry the risk of hypoglycemic episodes and weight gain, both of which are associated with poor cardiovascular outcomes (20). Therefore, longer-acting molecules that activate the insulin receptor without hypoglycemia would be helpful in the treatment of diabetes.
Antibodies have been shown to activate the INSR, including both spontaneously occurring human autoantibodies and mouse monoclonal antibodies (21–23). In humans, autoantibodies to the INSR typically bind at the insulin binding site (the orthosteric site). In most cases, these antibodies block insulin binding, causing severe insulin resistance and diabetes (24–27). However, it has been reported that in some individuals, orthosteric INSR autoantibodies mimic insulin and stimulate the INSR, causing hypoglycemia (26,28–30).
Orthosteric antibodies that mimic ligand signaling have also been reported for other receptors (31–34). It has also been reported that allosteric antibodies, antibodies that do not bind at the ligand binding site of receptors, can activate cell signaling (35). In theory, these allosteric antibodies have the potential to activate receptors more selectively than either orthosteric antibodies or the natural ligand itself, in that they do not recognize the binding determinants within a receptor that may cross-react with multiple ligands (e.g., the INSR is activated not only by insulin but also by IGFs). Allosteric regulation of the insulin receptor by glucose and peptides has been described previously (36–39). It is possible, therefore, that allosteric antibodies to receptors, such as the INSR, could be generated and be of benefit for the treatment of disease, including diabetes. To date, therapeutic allosteric antibodies to the INSR have not been reported.
To identify such antibodies, we selectively screened human phage display libraries for allosteric antibodies that activated the INSR. We selected one such allosteric antibody, XMetA, for further characterization both in vitro and in vivo. In cultured cells, XMetA activated INSR signaling and promoted glucose uptake. In diabetic mice, XMetA normalized fasting blood glucose for 6 weeks without hypoglycemia and improved metabolic parameters of diabetes.
RESEARCH DESIGN AND METHODS
XMetA discovery.
The extracellular domain of the human insulin receptor (hINSR; R&D Systems, Minneapolis, MN) was biotinylated with Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) according to the manufacturer’s protocol. To obtain allosteric antibodies to the INSR, panning and subsequent screening were performed with the biotinylated extracellular domain of the hINSR maintained in the presence of saturating insulin (10 μmol/L; Sigma-Aldrich, St. Louis, MO) to block the orthosteric binding site. This biotinylated receptor:ligand complex was immobilized on streptavidin-coated magnetic Dynabeads M-280 (Invitrogen Dynal AS, Oslo, Norway) and panned against two scFv antibody phage display libraries (XOMA [US], LLC, Berkeley, CA; BioInvent, Lund, Sweden), using standard methods (40,41). After each round of panning, phage were deselected against streptavidin-coated magnetic Dynabeads M-280 to remove nonspecific phage antibodies. After three rounds of panning and deselection, bead-bound phage were eluted and used to infect TG1 bacterial cells (Stratagene, La Jolla, CA). Phage were then rescued with helper phage M13KO7 (New England Biolabs, Ipswich, MA). Individual colonies were picked and grown in 96-well plates used to generate bacterial periplasmic extracts according to standard methods (40,41). The lysate supernatants were assayed for INSR binding by flow cytometry (vide infra). For this purpose, CHO cells that were transfected with either the hINSR (CHO-hINSR) or mouse INSR (CHO-mINSR) were used. Both INSR transfected cell lines had ∼250,000 receptors per cell compared with the untransfected cells, which had less than 5,000 INSR per cell as determined by flow cytrometry (similar numbers of IGF-IR were expressed on CHO-hIGF-IR cells; vide infra) (42). The single chain fragment (scFv) with the highest affinity was reformatted to a fully human IgG2a monoclonal antibody, XMetA (40,41).
Binding of XMetA to INSR.
For flow cytometry, cells (2 × 106/mL) were washed and resuspended in PBS with 0.5% fatty acid-free bovine serum albumin and 0.1% sodium azide (Fluorescence-activated cell sorter buffer; Invitrogen, Carlsbad, CA). Either XMetA or an antikeyhole limpet hemocyanin IgG2a isotype control antibody (XOMA [US]) was diluted in FACS buffer and incubated with cells at 4°C for 60 min. Cells were then washed once and resuspended in Alexa Fluor 647–conjugated goat anti-human IgG (1:200; Invitrogen). The cells were incubated for 30 min at 4°C, washed twice, and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Measurement of insulin binding affinity.
The effect of XMetA on the binding of insulin to the INSR was assessed in an equilibrium assay under conditions of a saturating antibody concentration (70 nmol/L). Human insulin (80 pmol/L; Sigma-Aldrich) and XMetA or isotype control antibody were incubated at 5°C with increasing concentrations of CHO-hINSR cells for 18 h. Cells were pelleted by centrifugation, and the amount of free insulin in solution was measured by immunofluorescence using a KinExA instrument (43). Insulin concentration data were curve-fit using KinExA software to yield the relative change in insulin binding affinity.
Insulin and IGF-I receptor signaling in cultured cells.
CHO cells at 37°C expressing mouse or human INSR or IGF-IR were incubated in serum-free culture medium with increasing concentrations of insulin, control antibody, or XMetA for 10 min. Total Akt and Akt phosphorylated at Ser473 and total Erk1/2 and Erk1/2 phosphorylated at Thr202/Tyr204; Thr185/Tyr187 were measured by enzyme-linked immunosorbent assay (ELISA; Meso Scale Discovery, Gaithersburg, MD). To evaluate the effect of XMetA on the hINSR and mINSR autophosphorylation, CHO cells were incubated with increasing concentrations of insulin, control antibody, or XMetA for 10 min and phosphotyrosine content of the INSR measured by ELISA (Millipore, Billerica, MA). To determine the effect of XMetA on autophosphorylation of the IGF-IR, CHO cells transfected with the human IGF-IR that had ∼250,000 receptors per cell were used (28). Cells were preincubated at 37°C in serum-free medium with a saturating concentration of XMetA (33 nmol/L) for 15 min followed by a 10-min incubation at 37°C with IGF-I (100 nmol/L). Tyrosine-phosphorylated INSR or IGF-IR was determined by ELISA (Millipore).
Glucose transport and proliferation assays.
To measure 2-deoxy-glucose uptake, 3T3 cells expressing hINSR (3T3-HIR cells; ∼1 × 106 hINSR/cell) were incubated in serum-free medium for 1 h with increasing concentrations of XMetA, isotype control antibody, or insulin. [3H]-2-deoxy-d-glucose was then added for 20 min, and its uptake was measured (28). MCF-7 cells (ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle's medium containing glucose at 4.5 g/L supplemented with 10% FBS and 2 mmol/L glutamine (Invitrogen) for normal maintenance. For the proliferation assay, cells (1 × 104) were seeded in 96-well white opaque microtiter plates and allowed to reattach for 24 h. After 24 h, cells were washed twice with prewarmed PBS and incubated in Dulbecco’s modified Eagle's medium containing glucose at 1 g/L and no phenol red, supplemented with 0.1% FBS and 2 mmol/L glutamine (Invitrogen; “starvation media”). XMetA, isotype control antibody, or insulin (Sigma-Aldrich) was added to the cells for a further 24 h and cell proliferation was measured using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI).
Mouse model of diabetes.
Animal experiments were approved by the XOMA (US) Institutional Animal Care and Use Committee (IACUC) and performed in accordance with IACUC guidelines. All animals were maintained in a pathogen-free environment and allowed free access to food and water. Six- to eight-week-old male ICR mice (Harlan, Indianapolis, IN) were randomly divided into groups administered streptozotocin (STZ; Sigma) or treated with citrate buffer. Buffer-treated (control) mice were fed a standard chow diet (Harlan), and diabetic animals were fed a 40 kcal% fat, 35 kcal% sucrose diet (Research Diets, New Brunswick, NJ) on arrival (44). STZ was dissolved in citrate buffer and injected intraperitoneally (40 mg/kg) for 5 consecutive days during the third week after arrival. After an additional week, mice were divided into groups (n = 8) and treated twice weekly, intraperitoneally, with XMetA or an antikeyhole limpet hemocyanin isotype control antibody, as indicated. Blood glucose was measured using the α-TRAK Blood Glucose Monitoring System (Abbott, Chicago, IL), and hemoglobin A1c was evaluated using the A1CNow platform (Bayer HealthCare, Tarrytown, NY). After ∼6 weeks of dosing, mice were killed after an overnight fast and terminal plasma was collected by cardiac puncture. Plasma insulin and C-peptide were measured by ELISA (Alpco Diagnostics, Salem, NH). XMetA was determined not to cross-react with capture or detection antibodies in these systems (data not shown). β-Hydroxybutyrate, cholesterol, triglycerides, and free fatty acids were determined by standard colorimetric methods (Wako Chemicals, Richmond, VA).
Glucose and insulin tolerance tests.
For glucose tolerance tests, mice were fasted overnight for 14 h followed by a glucose challenge (1 g/kg; Mediatech, Manassas, VA) by intraperitoneal injection or oral gavage, as indicated. Whole venous blood was obtained from the tail vein at 0, 15, 30, 60, and 120 min after challenge and evaluated for blood glucose. Insulin tolerance tests were carried out after a 4-h fast in animals by administering insulin (0.75 units/kg; Roche Diagnostics) intraperitoneally and measuring venous blood for glucose at 0, 30, 60, and 120 min after insulin challenge.
Statistical procedures.
All values, unless otherwise indicated, are expressed as mean ± SEM. Statistical analyses were carried out using a two-tailed Student unpaired t test using Prism 5.0 graphing software (GraphPad, La Jolla, CA).
RESULTS
XMetA binds to INSR at an allosteric site.
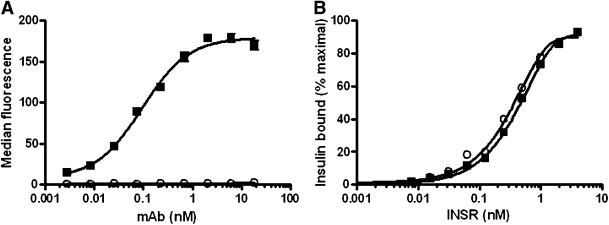
Allosteric antibodies to the INSR were identified by screening phage display libraries for human scFv fragments that bound the insulin-INSR complex. The highest affinity scFv fragment was then reformatted into a fully human IgG2 monoclonal antibody, XMetA. By flow cytometry, we determined that XMetA, but not an isotype control monoclonal antibody, specifically bound to CHO cells expressing the human INSR (CHO-hINSR) with an half-maximal effective concentration (EC50) of 0.10 nmol/L (Fig. 1A). With the use of KinExA methodology to determine the binding affinity of XMetA to the hINSR (43), XMetA bound to the hINSR with a KD of 0.040 nmol/L (data not shown). Neither XMetA nor the control antibody altered the affinity of insulin binding to the hINSR in the absence of antibodies (KD of 0.17 nmol/L) (Fig. 1B). XMetA also had no effect on the binding of labeled insulin to the INSR on these same cells by flow cytometry (data not shown). These data demonstrate, therefore, that XMetA binds to hINSR with high affinity, and this binding is to an allosteric site, which is distinct from the orthosteric insulin binding site.
FIG. 1.
XMetA binds to the INSR at an allosteric site. A: CHO-hINSR cells were incubated with increasing concentrations of either XMetA (■) or isotype control antibody (○) and antibody binding measured by flow cytometry (n = 4). B: Increasing concentrations of CHO-hINSR cells were incubated with 80 pmol/L insulin and either 70 nmol/L XMetA (■) or isotype control antibody (○). Insulin binding to the INSR was determined by KinExA (n = 3). mAb, monoclonal antibody.
XMetA is a partial agonist of INSR signaling that selectively activates the PI3K/Akt pathway.
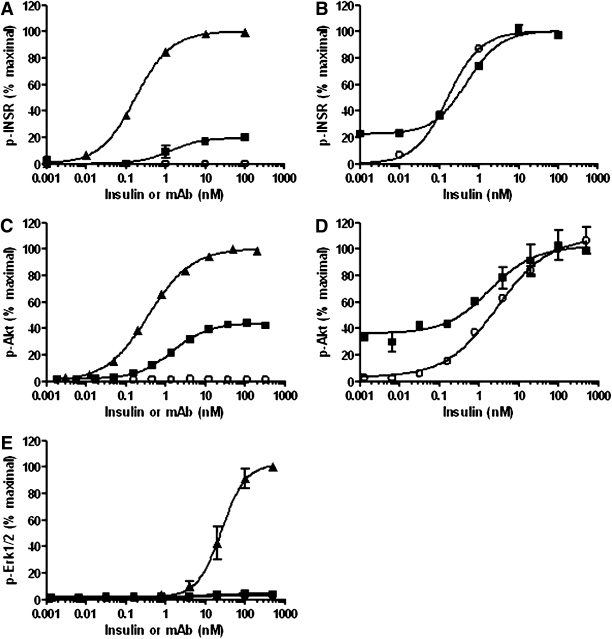
The ability of XMetA to activate the hINSR was evaluated in CHO-hINSR cells. We first studied hINSR autophosphorylation. Insulin activated this function with an EC50 of 0.18 nmol/L (Fig. 2A). XMetA activated INSR autophosphorylation with an EC50 of 1.3 nmol/L and maximal activation of ∼20% that of insulin, indicating that XMetA is a partial agonist of the hINSR. An isotype control antibody was without effect. When XMetA was used at a maximally stimulating concentration, insulin was still able to fully activate the hINSR with similar sensitivity (Fig. 2B). This observation strongly supports the notion that XMetA binds to the hINSR at an allosteric site and does not interfere with insulin binding its site. Similarly, XMetA, but not the control antibody, stimulated the phosphorylation of Akt, a major intracellular mediator of INSR-dependent glucoregulatory signaling, with an EC50 of 1.1 nmol/L (Fig. 2C). Maximal activation was ∼40% that of insulin, further demonstrating that XMetA is a partial agonist of the INSR. As with INSR autophosphorylation, when XMetA was used at a maximally stimulating concentration, insulin was still able to fully phosphorylate Akt with similar sensitivity (Fig. 2D). In contrast with insulin, XMetA did not stimulate the phosphorylation of Erk, which mediates INSR-dependent mitogenic properties (Fig. 2E), nor did it affect the capacity of insulin to phosphorylate Erk (data not shown).
FIG. 2.
XMetA is a partial agonist of the INSR that selectively activates the PI3K/Akt pathway. A: CHO-hINSR cells were incubated with increasing concentrations of either XMetA (■), isotype control antibody (○), or insulin (▲), and INSR autophosphorylation was measured by ELISA (n = 3). B: CHO-hINSR cells were incubated with either 33 nmol/L XMetA (■) or isotype control antibody (○) with increasing concentrations of insulin. INSR autophosphorylation was then measured (n = 3). C: CHO-hINSR cells were incubated with increasing concentrations of XMetA (■), isotype control antibody (○), or insulin (▲), and Akt phosphorylation was measured by ELISA (n = 3). D: CHO-hINSR cells were incubated with either 33 nmol/L XMetA (■) or isotype control antibody (○) with increasing concentrations of insulin. Akt phosphorylation was then measured (n = 3). E: CHO-hINSR cells were incubated with increasing concentrations of XMetA (■), isotype control antibody (○), or insulin (▲), and extracellular signal–related kinase (Erk)1/2 phosphorylation was measured by ELISA (n = 3). mAb, monoclonal antibody.
XMetA does not activate the IGF-IR.
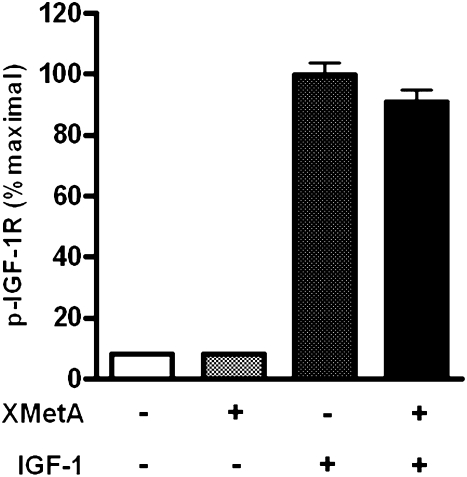
The IGF-IR has structural and functional similarity to INSR (45). We therefore investigated whether XMetA would activate the IGF-IR. For this purpose, we used CHO cells that expressed the human IGF-IR (CHO-hIGF-IR). Under conditions in which XMetA maximally activated INSR autophosphorylation in CHO-hINSR cells (Fig. 2A), XMetA neither directly activated autophosphorylation of the IGF1-R nor influenced the ability of the ligand, IGF-I, to activate this function (Fig. 3).
FIG. 3.
XMetA does not activate the IGF-IR. CHO-hIGF-IR cells were incubated with either 33 nmol/L XMetA or isotype control antibody in the presence or absence of 100 nmol/L IGF-I. IGF-IR autophosphorylation was measured by ELISA (n = 3). p, phosphorylation.
XMetA promotes glucose uptake but not cell growth.
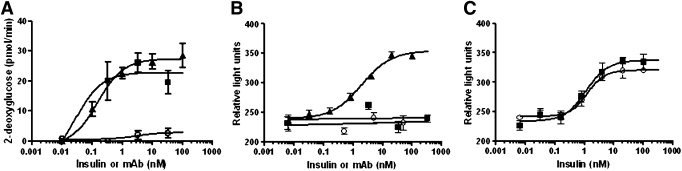
A major metabolic function of insulin is to enhance glucose transport. Accordingly, the uptake of 2-deoxy-d-glucose was analyzed in 3T3-HIR cells, a cell line that is known to be responsive to insulin (28). Insulin stimulated 2-deoxy-d-glucose uptake with an EC50 of 0.15 nmol/L. XMetA stimulated this function in a manner similar to that of insulin, whereas the control antibody was without effect (Fig. 4A). These data suggest that the lesser effects of XMetA on receptor phosphorylation may not fully indicate the effect of XMetA on insulin action.
FIG. 4.
XMetA promotes glucose uptake, but not cell growth. A: 3T3 cells expressing hINSR were incubated with increasing concentrations of either XMetA (■), isotype control antibody (○), or insulin (▲), and 2-deoxy-d-glucose uptake was measured (n = 5). B: MCF-7 cells were incubated with increasing concentrations of XMetA (■), isotype control antibody (○), or insulin (▲), and cell proliferation was determined by CellTiter Glo assay (n = 6). C: MCF-7 cells were incubated with either 33 nmol/L XMetA (■) or isotype control antibody (○) with increasing concentrations of insulin. Cell proliferation was then measured (n = 3). mAb, monoclonal antibody.
In addition to its metabolic effects, insulin, via its own receptor, stimulates cell growth, in particular the growth of cancer cells (46). MCF-7 human breast cancer cells, which express the hINSR, have been extensively used to study this effect of insulin (18,47,48). In these cells, insulin stimulated growth with an EC50 of 1.9 nmol/L (Fig. 4B). In contrast with insulin, neither XMetA nor the control antibody stimulated the growth of MCF-7 cells. Moreover XMetA did not potentiate the effect of insulin on cell proliferation (Fig. 4C). Similar results were obtained using methods that evaluate DNA content as a surrogate for proliferation (data not shown).
XMetA binds to and activates the mouse INSR.
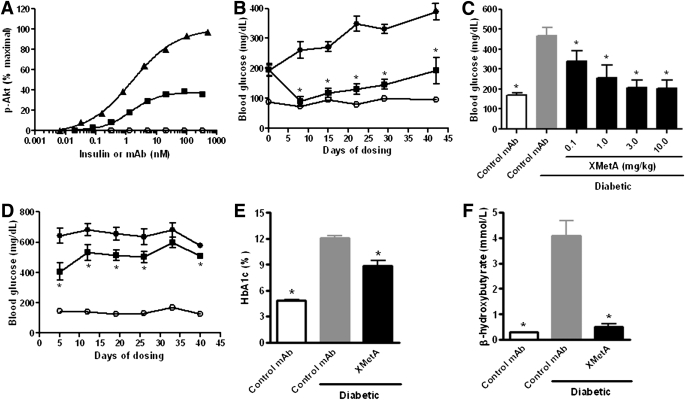
XMetA was next evaluated for its ability to bind to and activate the mINSR. For these studies we used CHO cells that expressed the mINSR (CHO-mINSR). By flow cytometry, we determined that XMetA, but not the isotype control antibody, specifically bound to CHO-mINSR cells with an EC50 of 0.085 nmol/L (data not shown), a value similar to that of its binding to CHO-hINSR cells. In CHO-mINSR cells, insulin stimulated the phosphorylation of Akt with an EC50 of 1.7 nmol/L. XMetA, but not the control antibody, stimulated the phosphorylation of Akt with a maximal effect of ∼40% that of insulin, with an EC50 of 1.4 nmol/L (Fig. 5A). These data indicated, therefore, that diabetic mice could be used to study the effects of XMetA on metabolic regulation.
FIG. 5.
XMetA improves hyperglycemia and other metabolic markers of disease in diabetic mice. A: CHO-mINSR cells were incubated with increasing concentrations of XMetA (■), isotype control antibody (○), or insulin (▲), and Akt phosphorylation was measured by ELISA (n = 3). B: Fasting blood glucose measurements were obtained weekly for 6 weeks from control mice treated with 10 mg/kg isotype control antibody (○) and diabetic mice treated with either 10 mg/kg XMetA (■) or isotype control antibody (●). C: After 3 weeks of treatment, fasting blood glucose was measured in control mice treated with 10 mg/kg isotype control antibody (white bar), diabetic mice treated with 10 mg/kg isotype control antibody (gray bar), and diabetic mice treated with the indicated doses of XMetA (black bars). D: Nonfasted blood glucose measurements were obtained weekly for 6 weeks from control mice treated with 10 mg/kg isotype control antibody (○) and diabetic mice treated with either 10 mg/kg XMetA (■) or isotype control antibody (●). After 6 weeks of treatment, blood hemoglobin A1c (E) and nonfasted plasma β-hydroxybutyrate (F) were measured in control mice treated with 10 mg/kg isotype control antibody (white bar) and diabetic mice treated with either 10 mg/kg isotype control antibody (gray bar) or XMetA (black bar). Values shown are mean ± SEM. *P < 0.05 for diabetic mice treated with XMetA compared with isotype control; n = 8 mice/group. mAb, monoclonal antibody.
XMetA improves fasting blood glucose levels in diabetic mice.
To evaluate the in vivo activity of XMetA, we used an animal model of insulinopenic, insulin-resistant diabetes, the multi-low dose STZ, high-fat diet (MLDS/HFD) mouse (44). Ten days after the last dose of STZ, blood glucose levels after a 14-h fast were elevated to ∼200 mg/dL, and these levels increased over the course of the 6-week study (Fig. 5B). In contrast, nondiabetic control animals maintained fasting glucose levels of ∼100 mg/dL. XMetA was administered by intraperitoneal injection twice weekly to the diabetic mice. Seven days after treatment, fasting blood glucose levels in the XMetA-treated diabetic mice were near normal and remained near normal for up to 28 days, but were slightly elevated at 42 days. At this time, anti-human IgG antibodies were detected in the treated mice (data not shown). These antibodies likely enhanced XMetA clearance, causing this elevation in blood glucose. An effect of XMetA was detected at doses as low as 0.1 mg/kg (Fig. 5C). XMetA maximally improved fasting blood glucose levels at a dose of 1.0 mg/kg. At this and higher doses of XMetA, there was no evidence of hypoglycemia.
XMetA improves nonfasting blood glucose levels in diabetic mice.
Glucose levels in diabetic mice allowed free access to food and water were also measured. Nondiabetic control animals maintained glucose levels in the range of 150 mg/dL (Fig. 5D). Diabetic animals had glucose values in the range of 600 mg/dL. Treatment with XMetA lowered nonfasted glucose values in diabetic mice, but in contrast with the results observed in fasted diabetic mice, XMetA did not normalize nonfasted glucose values.
XMetA improves metabolic markers of diabetes.
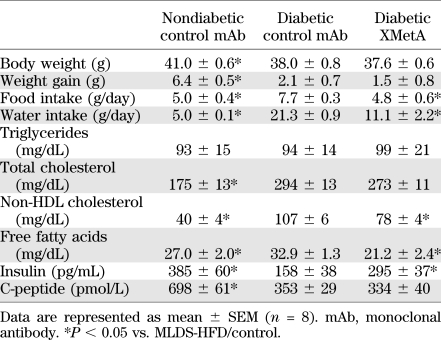
Various parameters in the mice were studied at the end the 6 weeks of treatment. In addition to improving fasting blood glucose to near normal levels and reducing nonfasted glucose levels, treatment with XMetA improved other metabolic indices in the diabetic animals. Consistent with the decrease in glucose levels, XMetA had a major effect on hemoglobin A1c (Fig. 5E). The diabetic animals were markedly ketotic as measured by β-hydroxybutyrate; XMetA normalized this value (Fig. 5F). XMetA treatment increased insulin levels (295 ± 37 vs. 158 ± 38 pg/mL, P < 0.05) without increasing the level of C-peptide (334 ± 40 vs. 353 ± 29 pmol/L) (Table 1). Diabetes reduced weight gain in diabetic animals, and XMetA treatment of diabetic animals did not change this parameter. However, other manifestations of diabetes were improved by XMetA. Food intake was decreased (4.8 ± 0.6 vs. 7.7 ± 0.3 g/day, P < 0.05), and water intake was decreased (11.1 ± 2.2 vs. 21.3 ± 0.9 g/day, P < 0.05). In addition, XMetA also improved non-HDL cholesterol (78 ± 4 vs. 107 ± 6 mg/dL, P < 0.05) and free fatty acids (21.2 ± 2.4 vs. 32.9 ± 1.3 mg/dL, P < 0.05).
TABLE 1.
Metabolic profile of diabetic MLDS/HFD mice treated with XMetA
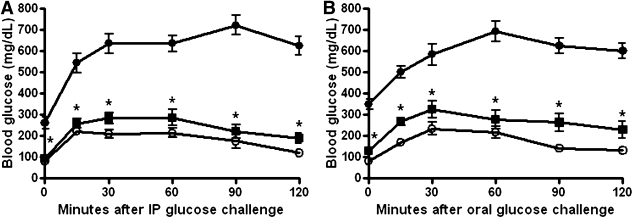
XMetA improves glucose tolerance in diabetic mice.
After 3 weeks of XMetA treatment, fasted diabetic animals underwent glucose tolerance tests, with either intraperitoneal (Fig. 6A) or oral (Fig. 6B) glucose. Diabetic animals developed markedly elevated glucose levels, reaching 600 mg/dL or greater. In contrast, during both types of glucose tolerance tests, animals treated with XMetA maintained near normal blood glucose concentrations.
FIG. 6.
XMetA improves glucose tolerance in diabetic mice. A: After 3 weeks of treatment, glucose was administered intraperitoneally (IP) at 1 g/kg to fasted mice. Blood glucose levels were measured for 120 min in control mice treated with 10 mg/kg (○) and diabetic mice treated with either 10 mg/kg XMetA (■) or isotype control antibody (●). B: After 3 weeks of treatment, glucose was administered orally at 1 g/kg to fasted mice. Blood glucose levels were measured for 120 min in control mice treated with 10 mg/kg isotype control antibody (○) and diabetic mice treated with either 10 mg/kg XMetA (■) or isotype control antibody (●). Values shown are mean ± SEM. *P < 0.05 for diabetic mice treated with XMetA compared with isotype control antibody; n = 8 mice/group.
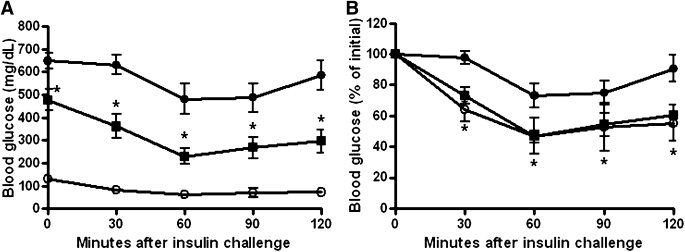
XMetA improves insulin tolerance in diabetic mice.
We next studied the effect of XMetA administration on the glucose response to exogenous insulin administration. After 5 weeks of treatment, animals were given intraperitoneal exogenous insulin, and the fall in blood glucose was measured for up to 120 min (Fig. 7A). Control, diabetic, and diabetic XMetA-treated animals all responded to insulin with a fall in blood glucose. When expressed as the percent change from initial glucose levels, the response to insulin in the diabetic animals was blunted compared with normal mice. This blunted response was corrected by XMetA treatment, suggesting that XMetA effects in this context are additive (Fig. 7B).
FIG. 7.
XMetA improves insulin tolerance in diabetic mice. A: After 5 weeks of treatment, insulin was administered intraperitoneally at 0.75 units/kg. Blood glucose levels were measured for 120 min in control mice treated with 10 mg/kg isotype control antibody (○) and diabetic mice treated with either 10 mg/kg XMetA (■) or isotype control antibody (●). B: Data normalized to preinsulin glucose values. Values shown are mean ± SEM. *P < 0.05 for diabetic mice treated with XMetA compared with isotype control antibody; n = 8 mice/group.
DISCUSSION
In the current study, we characterized XMetA, a novel fully human monoclonal antibody that is a high-affinity allosteric agonist of the INSR. To identify this type of antibody, we used the insulin:INSR complex to screen phage libraries. This approach reduced the potential for obtaining antibodies that bound to the orthosteric site of the INSR because the latter site was occupied by its ligand, insulin. Two lines of evidence indicated that the binding of XMetA to the INSR was allosteric and not orthosteric. First, although XMetA bound directly to the INSR, it did not compete with insulin for receptor binding. Second, functional studies in cells and metabolic studies in animals demonstrated that XMetA did not interfere with the actions of insulin. A major finding was that XMetA activated the insulin receptor in a manner that was different than insulin, an effect likely a result of a distinct structural state of the INSR induced by XMetA binding to its allosteric site.
After insulin binds to the INSR, it stimulates receptor autophosphorylation. XMetA also stimulated this function, but compared with insulin, it acted as a partial agonist. In contrast with its effects on the INSR, and unlike insulin itself (46), XMetA did not activate the IGF-IR. The metabolic effects of the INSR are known to occur primarily through the PI3K/Akt pathway (17). XMetA was also a partial agonist of the PI3K/Akt pathway as measured by Akt phosphorylation. XMetA, like insulin, activated glucose transport but, unlike insulin, did not stimulate the growth of cancer cells. Accordingly, XMetA did not activate Erk, which mediates insulin-dependent mitogenic signaling (47,48). Therefore, XMetA, which bound allosterically to the INSR, recapitulated glucoregulatory functions of insulin, without mimicking insulin-promoted cell proliferation.
XMetA bound to and activated both the human and mouse INSR with similar dynamics, thus allowing in vivo studies in the MLDS/HFD mouse. The MLDS/HFD mouse is a commonly used model of insulin-resistant, insulinopenic diabetes (44,49). In these animals, XMetA normalized both fasting glucose levels and glucose tolerance. Nonfasted glucose levels, however, were improved, but not fully normalized. The reasons for this are currently unclear. One possibility is that, as a partial agonist that does not activate Erk, XMetA may not activate insulin-dependent gene expression in the same manner as insulin itself. Additional studies are needed to fully understand this phenomenon. Nevertheless, the improvements in glucose resulted in a significant improvement in hemoglobin A1c. XMetA also improved other indices of diabetes including hyperphagia and polydipsia, without significantly impacting body weight in this model. Efficacy, as measured by fasting blood glucose levels, was observed at an intraperitoneal dose as low as 0.1 mg/kg and was maximal at 1.0 mg/kg. Preliminary studies suggest that subcutaneous administration of XMetA for up to 3 weeks is equally as effective as intraperitoneal dosing in MLDS/HFD mice (V.B. and A.L., unpublished data).
Of major interest was the observation that XMetA did not cause hypoglycemia. XMetA was not associated with hypoglycemia even when administered at doses that were 10-fold above the minimum dose required for complete efficacy. The reasons for this positive outcome are not fully understood at this time. One possibility is that, as a partial agonist of the INSR, the hypoglycemic potential of XMetA is lower than that of insulin and does not overwhelm the endogenous compensatory mechanisms that prevent hypoglycemia. Another possibility is that XMetA tissue–specific distribution and/or activity differs from than that of insulin. Further studies may help fully understand this phenomenon.
As an allosteric activator of the INSR, XMetA allowed the binding of insulin and subsequent insulin signaling. When an insulin bolus was given to diabetic mice, the effect of insulin, as measured by kinetics of glucose lowering, was diminished relative to that observed in normal mice. It is noteworthy that XMetA normalized these kinetics, most likely by its ability to correct many of the metabolic abnormalities associated with the diabetic state. These studies demonstrate, therefore, that XMetA improves the response to an insulin bolus in diabetic mice. This outcome raises the interesting possibility that XMetA may be used in combination with short-acting insulins in diabetes.
In addition to positive effects on glucose metabolism in diabetic mice, XMetA improved other metabolic parameters. XMetA lowered plasma non-HDL cholesterol and free fatty acids and completely normalized hyperketonemia. The observed improvement in β-hydroxybutyrate was greater than the improvement in hemoglobin A1c, consistent with the observation that lower levels of INSR signaling are required to prevent diabetic ketoacidosis compared with that required for complete glycemic control (50). XMetA increased circulating insulin levels without a concomitant increase in C-peptide, suggesting that XMetA may affect insulin clearance by binding the INSR in the liver and other organs. Thus it is possible that the observed increase in circulating insulin contributes to the metabolic improvements described herein, in addition to the direct glucoregulatory activity of XMetA. Again, further studies may help fully understand the mechanisms whereby XMetA controls glucose and insulin metabolism.
In the treatment of diabetes, novel and improved therapeutic modalities for those individuals with impaired insulin secretory function would be helpful. Currently, long-acting basal insulins are given daily, or more often, and are associated with both hypoglycemia and weight gain (20). To more effectively control basal hyperglycemia, either very long acting insulins or other novel agents that stimulate the INSR could be of benefit. Therefore, a highly specific, ultra-long-acting activator of INSR, such as a monoclonal antibody, would represent a new paradigm in diabetes therapy. Herein, we demonstrate that XMetA, an allosteric activator of INSR both in vitro and in vivo, has the potential to normalize glycemic control in a model of insulinopenic diabetes without causing hypoglycemia or promoting weight gain. To our knowledge, XMetA is the first fully human monoclonal antibody to the INSR reported to correct hyperglycemia and improve diabetes in vivo. Fully human antibodies typically have a half-life greater than 1 week and relatively low immunogenicity in humans. Thus, a fully human monoclonal antibody that activates the INSR has the potential for dosing in humans at once weekly intervals or less. These observations suggest, therefore, that antibodies like XMetA, either alone or in combination with short acting insulins/oral agents, may represent a novel strategy for controlling blood glucose levels in diabetes.
ACKNOWLEDGMENTS
V.B., D.H.B., A.L., H.F.K., L.M.G., M.H., S.R.W., S.Z., A.J.N., R.L., L.W., N.L., X.W., D.C.-V., C.T., S.W., S.R.L., S.W., D.W., and J.A.C. are employees of XOMA (US), LLC. I.D.G. is a consultant to XOMA (US), LLC. No other potential conflicts of interest relevant to this article were reported.
V.B. designed the in vivo experiments, researched the data, and wrote the manuscript. I.D.G. wrote and edited the manuscript and contributed to the discussion. D.H.B., H.F.K., M.H., B.A.M., S.R.W., S.Z., L.W., S.D.W., N.L., X.W., D.C.-V., C.T., S.R.L., and S.W. researched the in vitro data. A.L., L.M.G., A.J.N., and S.R.L. researched the in vivo data. D.W. and M.L.W. edited the manuscript and contributed to the discussion. J.A.C. led the research team, designed the in vitro experiments, and edited the manuscript. V.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Patrick J. Scannon, XOMA (US), LLC, for critical reading of the manuscript and helpful comments.
REFERENCES
- 1.2011. 2011. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [Google Scholar]
- 2.Kahn CR, Flier JS, Bar RS, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 1976;294:739–745 [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. The insulin resistance syndrome. Curr Atheroscler Rep 2003;5:364–371 [DOI] [PubMed] [Google Scholar]
- 5.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am 2004;33:283–303 [DOI] [PubMed] [Google Scholar]
- 6.Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 1980;65:1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaven GM, Olefsky JM. The role of insulin resistance in the pathogenesis of diabetes mellitus. Adv Metab Disord 1978;9:313–331 [DOI] [PubMed] [Google Scholar]
- 8.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 1992;340:925–929 [DOI] [PubMed] [Google Scholar]
- 9.Lyssenko V, Almgren P, Anevski D, et al. Botnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 10.Chen YD, Jeng CY, Hollenbeck CB, Wu MS, Reaven GM. Relationship between plasma glucose and insulin concentration, glucose production, and glucose disposal in normal subjects and patients with non-insulin-dependent diabetes. J Clin Invest 1988;82:21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990;113:909–915 [DOI] [PubMed] [Google Scholar]
- 12.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 13.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006;7:85–96 [DOI] [PubMed] [Google Scholar]
- 15.Asano T, Fujishiro M, Kushiyama A, et al. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull 2007;30:1610–1616 [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med 2004;10:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes 2010;1:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009;25:41–49 [DOI] [PubMed] [Google Scholar]
- 19.Pollock RF, Erny-Albrecht KM, Kalsekar A, Bruhn D, Valentine WJ. Long-acting insulin analogs: a review of “real-world” effectiveness in patients with type 2 diabetes. Curr Diabetes Rev 2011;7:61–74 [DOI] [PubMed] [Google Scholar]
- 20.Niswender KD. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad Med 2011;123:17–26 [DOI] [PubMed] [Google Scholar]
- 21.Goldfine ID, Roth RA. Monoclonal antibodies to the insulin receptor as probes of insulin receptor structure and function. Horiz Biochem Biophys 1986;8:471–502 [PubMed] [Google Scholar]
- 22.Soos MA, Siddle K, Baron MD, et al. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J 1986;235:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddle K, Soos MA, O’Brien RM, Ganderton RH, Taylor R. Monoclonal antibodies as probes of the structure and function of insulin receptors. Biochem Soc Trans 1987;15:47–51 [DOI] [PubMed] [Google Scholar]
- 24.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141–153 [DOI] [PubMed] [Google Scholar]
- 25.Le Marchand-Brustel Y, Gorden P, Flier JS, Kahn CR, Freychet P. Anti-insulin receptor antibodies inhibit insulin binding and stimulate glucose metabolism in skeletal muscle. Diabetologia 1978;14:311–317 [DOI] [PubMed] [Google Scholar]
- 26.De Pirro R, Roth RA, Rossetti L, Goldfine ID. Characterization of the serum from a patient with insulin resistance and hypoglycemia. Evidence for multiple populations of insulin receptor antibodies with different receptor binding and insulin-mimicking activities. Diabetes 1984;33:301–304 [DOI] [PubMed] [Google Scholar]
- 27.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87–100 [DOI] [PubMed] [Google Scholar]
- 28.Brunetti A, Maddux BA, Wong KY, et al. Monoclonal antibodies to the human insulin receptor mimic a spectrum of biological effects in transfected 3T3/HIR fibroblasts without activating receptor kinase. Biochem Biophys Res Commun 1989;165:212–218 [DOI] [PubMed] [Google Scholar]
- 29.Zick Y, Rees-Jones RW, Taylor SI, Gorden P, Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem 1984;259:4396–4400 [PubMed] [Google Scholar]
- 30.Taylor SI, Grunberger G, Marcus-Samuels B, et al. Hypoglycemia associated with antibodies to the insulin receptor. N Engl J Med 1982;307:1422–1426 [DOI] [PubMed] [Google Scholar]
- 31.Chen CR, McLachlan SM, Rapoport B. Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 2007;148:2375–2382 [DOI] [PubMed] [Google Scholar]
- 32.Peter JC, Eftekhari P, Billiald P, Wallukat G, Hoebeke J. scFv single chain antibody variable fragment as inverse agonist of the beta2-adrenergic receptor. J Biol Chem 2003;278:36740–36747 [DOI] [PubMed] [Google Scholar]
- 33.Josephson K, Jones BC, Walter LJ, DiGiacomo R, Indelicato SR, Walter MR. Noncompetitive antibody neutralization of IL-10 revealed by protein engineering and x-ray crystallography. Structure 2002;10:981–987 [DOI] [PubMed] [Google Scholar]
- 34.Colwell NS, Blinder MA, Tsiang M, Gibbs CS, Bock PE, Tollefsen DM. Allosteric effects of a monoclonal antibody against thrombin exosite II. Biochemistry 1998;37:15057–15065 [DOI] [PubMed] [Google Scholar]
- 35.Cazorla M, Arrang JM, Prémont J. Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor. Br J Pharmacol 2011;162:947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Root-Bernstein R, Vonck J. Glucose binds to the insulin receptor affecting the mutual affinity of insulin and its receptor. Cell Mol Life Sci 2009;66:2721–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Meyts P, Gauguin L, Svendsen AM, et al. Structural basis of allosteric ligand-receptor interactions in the insulin/relaxin peptide family: implications for other receptor tyrosine kinases and G-protein-coupled receptors. Ann N Y Acad Sci 2009;1160:45–53 [DOI] [PubMed] [Google Scholar]
- 38.De Meyts P. The insulin receptor: a prototype for dimeric, allosteric membrane receptors? Trends Biochem Sci 2008;33:376–384 [DOI] [PubMed] [Google Scholar]
- 39.Jensen M, Hansen B, De Meyts P, Schäffer L, Ursø B. Activation of the insulin receptor by insulin and a synthetic peptide leads to divergent metabolic and mitogenic signaling and responses. J Biol Chem 2007;282:35179–35186 [DOI] [PubMed] [Google Scholar]
- 40.A. Aa CFB D.R. Burton, J.K. Scott, G.J. Silverman: Phage Display: A Laboratory Manual. Plainview, NY, Cold Spring Harbor Laboratory Press, 2001 [Google Scholar]
- 41.Aitken R. Methods in Molecular Biology. In Antibody phage display: methods and protocols. O'Brien PM, Ed. Totowa, NJ, Humana Press, 2001 [Google Scholar]
- 42.Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood 2003;102:2156–2164 [DOI] [PubMed] [Google Scholar]
- 43.Rathanaswami P, Babcook J, Gallo M. High-affinity binding measurements of antibodies to cell-surface-expressed antigens. Anal Biochem 2008;373:52–60 [DOI] [PubMed] [Google Scholar]
- 44.Arulmozhi DK, Kurian R, Bodhankar SL, Veeranjaneyulu A. Metabolic effects of various antidiabetic and hypolipidaemic agents on a high-fat diet and multiple low-dose streptozocin (MLDS) mouse model of diabetes. J Pharm Pharmacol 2008;60:1167–1173 [DOI] [PubMed] [Google Scholar]
- 45.Lawrence MC, McKern NM, Ward CW. Insulin receptor structure and its implications for the IGF-1 receptor. Curr Opin Struct Biol 2007;17:699–705 [DOI] [PubMed] [Google Scholar]
- 46.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology 2011;152:2546–2551 [DOI] [PubMed] [Google Scholar]
- 47.Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem 2008;114:38–44 [DOI] [PubMed] [Google Scholar]
- 48.Mayer D, Chantelau E. Treatment with insulin glargine (Lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: a pilot study on MCF-7 breast cancer cells. Arch Physiol Biochem 2010;116:73–78 [DOI] [PubMed] [Google Scholar]
- 49.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med 2005;22:359–370 [DOI] [PubMed] [Google Scholar]
- 50.Kreisberg RA. Diabetic ketoacidosis: new concepts and trends in pathogenesis and treatment. Ann Intern Med 1978;88:681–695 [DOI] [PubMed] [Google Scholar]