Abstract
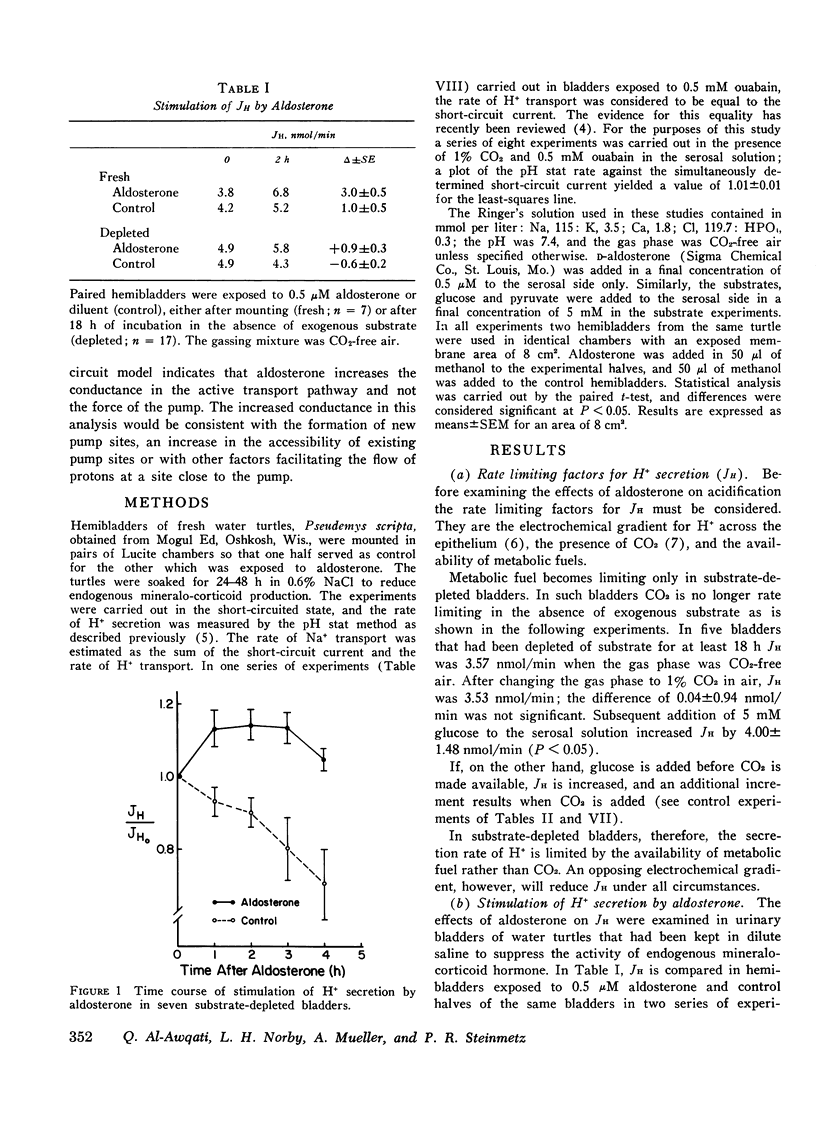
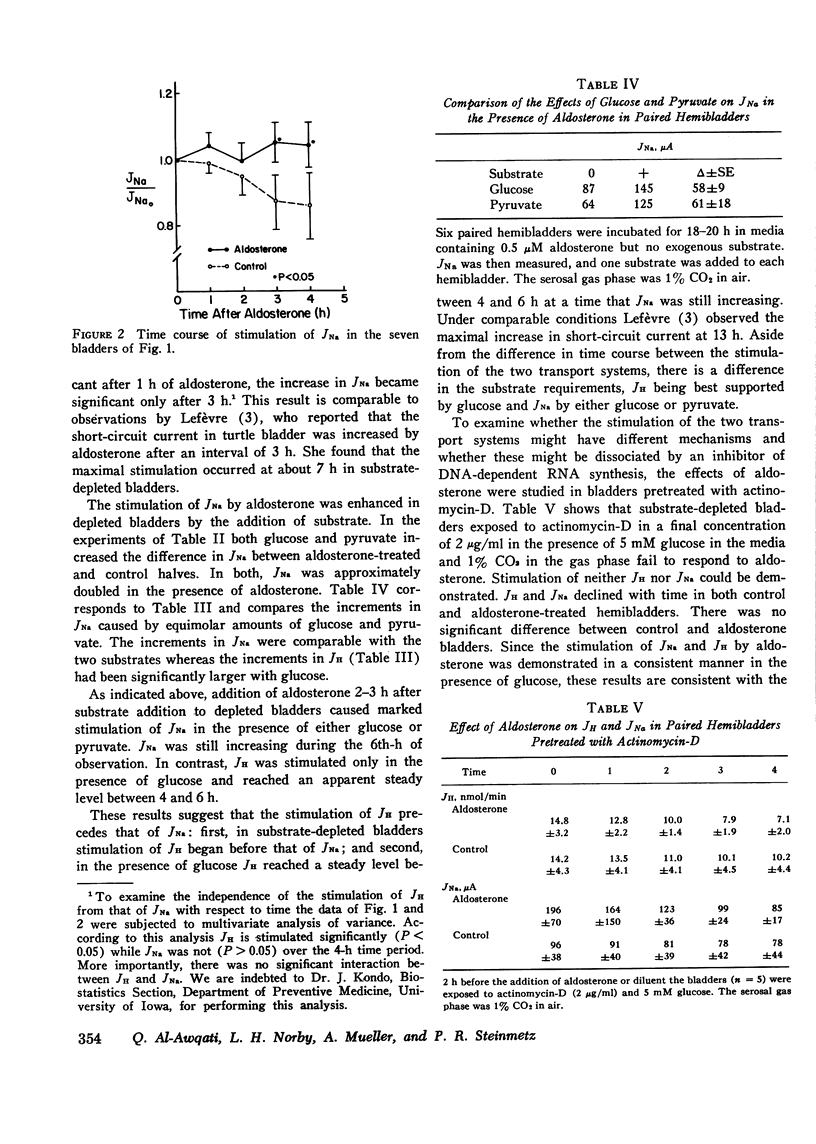
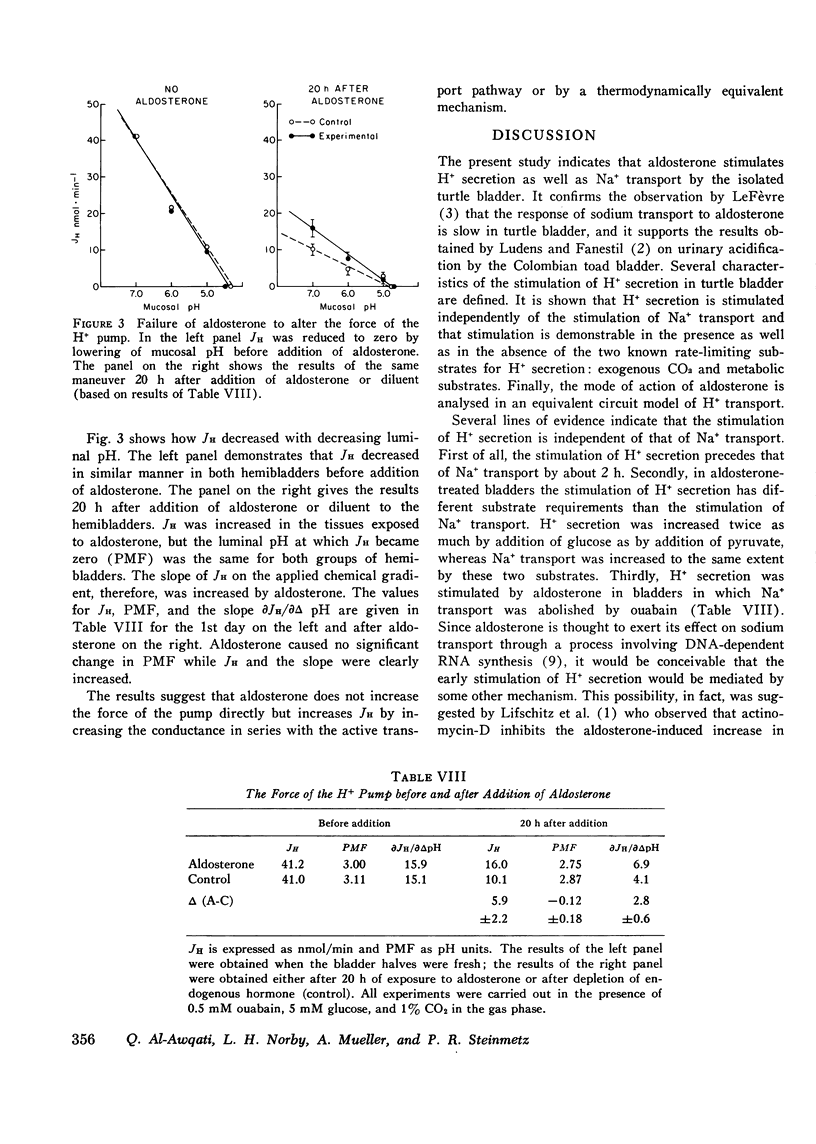
Aldosterone stimulates not only Na+ absorption but also urinary acidification. In this investigation the effects of aldosterone on H+ transport are examined in vitro in turtle bladder, a urinary membrane in which several of the factors controlling H+ transport have been defined. H+ transport was increased in bladder halves exposed to aldosterone compared to control halves. Stimulation of H+ secretion was observed as early as 1 h after addition of aldosterone and occurred before that of Na+ transport. In bladders depleted of endogenous substrate addition of glucose increased H+ transport more in aldosterone-treated halves (10.0+/-1.3 nmol/min) than in control halves (6.8+/-2.3). Addition of pyruvate failed to increase H+ transport (--0.3+/-0.7) in control halves but caused significant increments (2.4+/-0.5) in aldosterone-treated halves. In aldosterone-treated bladders glucose caused larger increments (16.5+/-2.7) in H+ transport than pyruvate (9.3+/-2.0) when halves of the same bladders were compared. Na+ transport, however, was equally increased by the two substrates. Despite the differences in time course and substrate requirements between the stimulation of H+ and Na+ transport, both increases were abolished by actinomycin-D. To examine the effect of aldosterone on the force of the H+ pump, protonmotive force, the pH gradient that would nullify the transport rate was determined with and without aldosterone. Aldosterone did not alter protonmotive force but significantly increased the slope of the H+ transport rate on the applied pH gradient. It is concluded that aldosterone stimulates H+ transport independently of Na+ transport. It increases the responsiveness of the transport rate to glucose and to a lesser extent pyruvate, an effect probably secondary to the increased transport rate. Equivalent circuit analysis indicates that aldosterone facilitates the flow of protons through the active transport pathway but does not increase the force of the pump.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civan M. M., Kedem O., Leaf A. Effect of vasopressin on toad bladder under conditions of zero net sodium transport. Am J Physiol. 1966 Sep;211(3):569–575. doi: 10.1152/ajplegacy.1966.211.3.569. [DOI] [PubMed] [Google Scholar]
- EDELMAN I. S., BOGOROCH R., PORTER G. A. ON THE MECHANISM OF ACTION OF ALDOSTERONE ON SODIUM TRANSPORT: THE ROLE OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1169–1177. doi: 10.1073/pnas.50.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig A., Caplan S. R. Energetics of active transport processes. Biophys J. 1968 Dec;8(12):1434–1457. doi: 10.1016/S0006-3495(68)86565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Allen J. E., Rasmussen H. Studies on the mechanism of action of aldosterone: hormone-induced changes in lipid metabolism. Biochemistry. 1971 Oct 12;10(21):3825–3831. doi: 10.1021/bi00797a004. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E. Effects of aldosterone on the isolated substrate-depleted turtle bladder. Am J Physiol. 1973 Nov;225(5):1252–1256. doi: 10.1152/ajplegacy.1973.225.5.1252. [DOI] [PubMed] [Google Scholar]
- Lifschitz M. D., Schrier R. W., Edelman I. S. Effect of actinomycin D on aldosterone-mediated changes in electrolyte excretion. Am J Physiol. 1973 Feb;224(2):376–380. doi: 10.1152/ajplegacy.1973.224.2.376. [DOI] [PubMed] [Google Scholar]
- Ludens J. H., Fanestil D. D. Aldosterone stimulation of acidification of urine by isolated urinary bladder of the Colombian toad. Am J Physiol. 1974 Jun;226(6):1321–1326. doi: 10.1152/ajplegacy.1974.226.6.1321. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. CO2 requirements for H+ secretion by the isolated turtle bladder. Am J Physiol. 1971 Jun;220(6):2051–2057. doi: 10.1152/ajplegacy.1971.220.6.2051. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Characteristics of hydrogen ion transport in urinary bladder of water turtle. J Clin Invest. 1967 Oct;46(10):1531–1540. doi: 10.1172/JCI105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Effect of luminal pH on ion permeability and flows of Na+and H+ in turtle bladder. Am J Physiol. 1971 Jun;220(6):1573–1580. doi: 10.1152/ajplegacy.1971.220.6.1573. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]


