Abstract
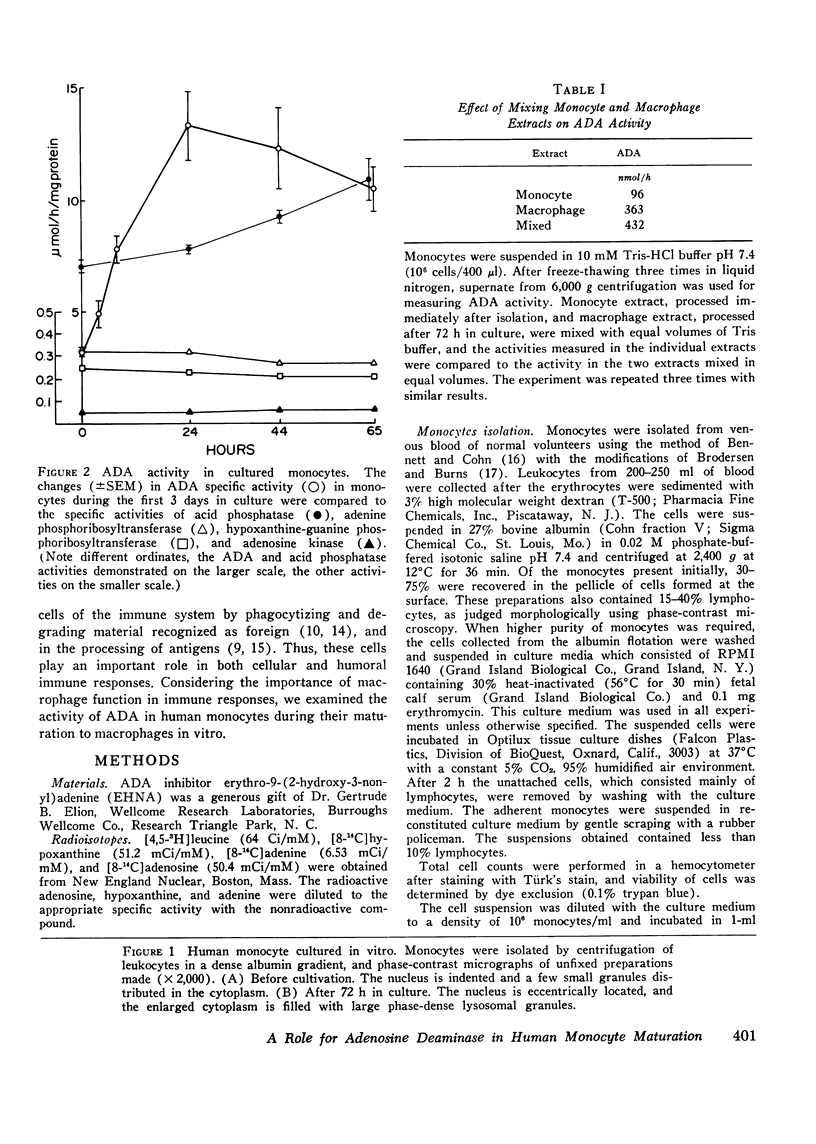
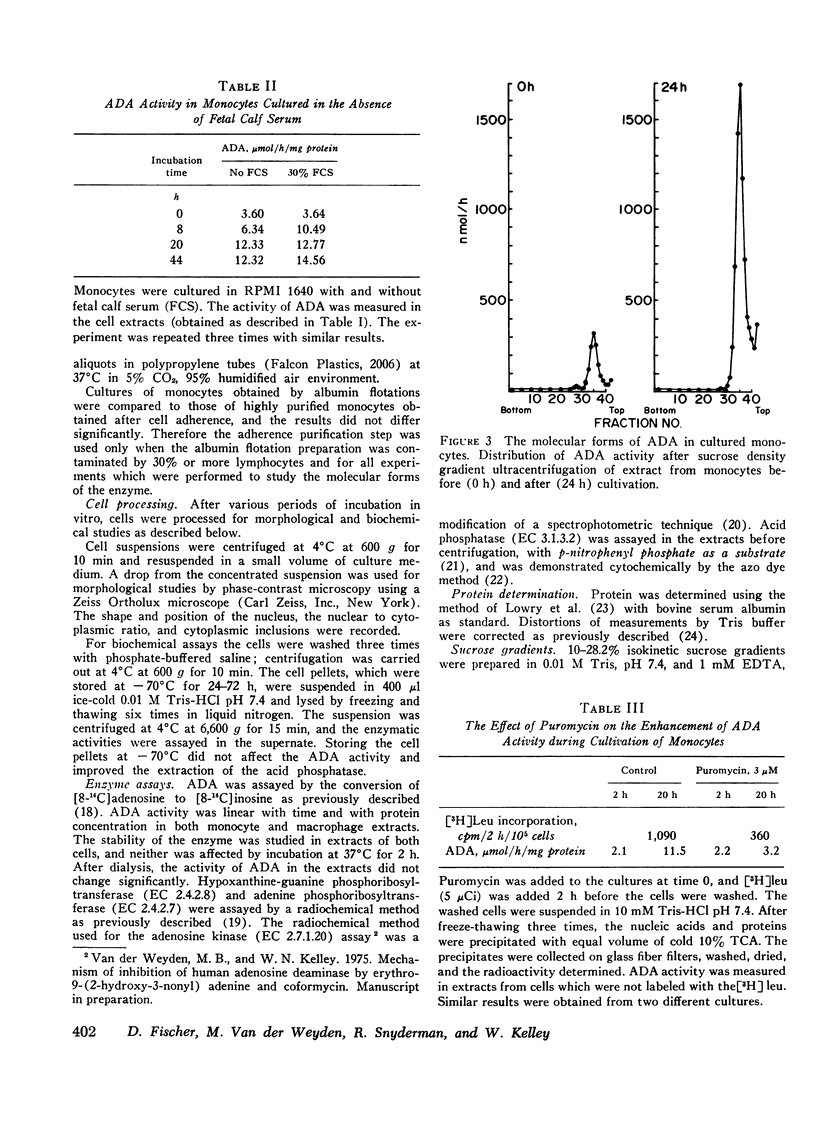
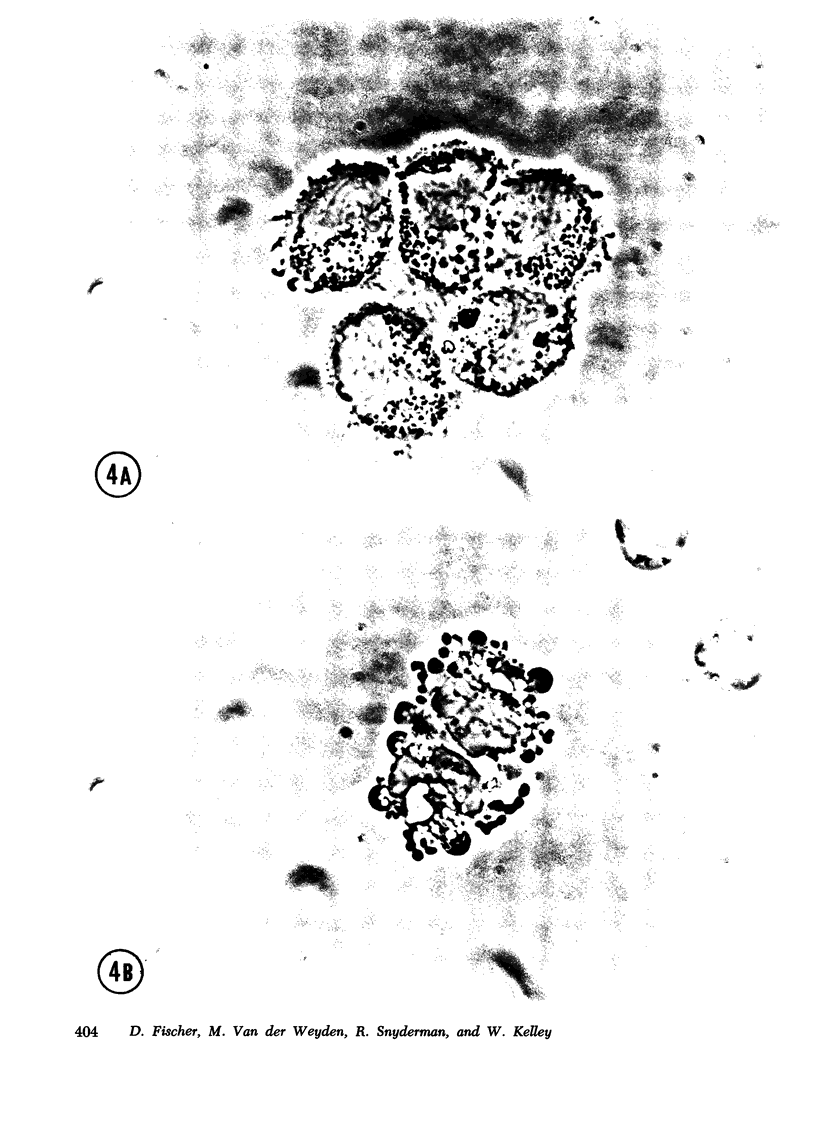
The occurrence of a deficiency of adenosine deaminase (ADA) activity in some patients with severe combined immunodeficiency suggests a possible relationship between the activity of ADA and the aberration of the immune system. To help delineate the function of ADA in the immune response we have examined its role in monocyte maturation. When incubated in vitro, peripheral blood monocytes transformed, within 3 days, to macrophagea as assessed by phase-contrast microscopy and an increase in the specific activity of the lysosomal enzyme acid phosphatase. The specific activity of ADA increased as much as ninefold, reaching a peak after the 1st day in culture, while the activities of other enzymes involved in the purine salvage pathway were not altered. Sucrose density ultracentrifugation of extracts prepared immediately after the isolation of monocytes revealed the presence of two forms of ADA with molecular weights of approximately 30,000 and 110,000. The increase in ADA specific activity during monocyte cultivation correlated with an increase in the activity of the smaller molecular species. A specific inhibitor ADA, erythro-9-(2-hydroxy-3-nonyl) adenine, prevented the increase in acid phosphatase activity, as well as the morphological changes associated with the monocyte maturation. These data suggest a role for ADA in monocyte to macrophage maturation. In view of the central role of macrophages in immune function, this observation may relate to the association of combined immunodeficiency and a deficiency of this enzyme.
Full text
PDF



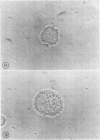
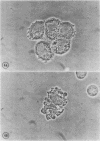
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen M. P., Burns C. P., Hoak J. C. The separation of human monocytes from blood including biochemical observations. Proc Soc Exp Biol Med. 1973 Dec;144(3):941–944. doi: 10.3181/00379727-144-37716. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Dissing J., Knudsen B. Adenosine-deaminase deficiency and combined immunodeficiency syndrome. Lancet. 1972 Dec 16;2(7790):1316–1316. doi: 10.1016/s0140-6736(72)92692-x. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Green H., Ishii K. On the existence of a guanine nucleotide trap, the role of adenosine kinase and a possible cause of excessive purine production in mammalian cells. J Cell Sci. 1972 Jul;11(1):173–177. doi: 10.1242/jcs.11.1.173. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Levytska V. Alterations in isozymes of adenosine deaminase during stimulation of human peripheral blood lymphocytes. Cell Immunol. 1974 Jun;12(3):387–395. doi: 10.1016/0008-8749(74)90095-1. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Two molecular forms interconvertible by purine ribonucleotides and phosphoribosylpyrophosphate. J Biol Chem. 1973 Sep 10;248(17):6035–6040. [PubMed] [Google Scholar]
- Ishii K., Green H. Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J Cell Sci. 1973 Sep;13(2):429–439. doi: 10.1242/jcs.13.2.429. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindberg B., Klenow H., Hansen K. Some properties of partially purified mammalian adenosine kinase. J Biol Chem. 1967 Feb 10;242(3):350–356. [PubMed] [Google Scholar]
- Manohar S. V., Lerner M. H., Rubinstein D. The metabolism of the erythrocyte. 18. Inhibition of nucleotide synthesis in human erythrocytes by adenosine. Can J Biochem. 1968 May;46(5):445–450. doi: 10.1139/o68-067. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Stafford D., Brown O. Resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1968 Aug;24(2):314–329. doi: 10.1016/0003-2697(68)90185-1. [DOI] [PubMed] [Google Scholar]
- Meuret G., Hoffmann G. Monocyte kinetic studies in normal and disease states. Br J Haematol. 1973 Mar;24(3):275–285. doi: 10.1111/j.1365-2141.1973.tb01652.x. [DOI] [PubMed] [Google Scholar]
- ROZENSZAJN L., MARSHAK G., EFRATI P. ACID PHOSPHATASE ACTIVITY IN NORMAL HUMAN BLOOD AND BONE MARROW CELLS AS DEMONSTRATED BY THE AZO DYE METHOD. Acta Haematol. 1963 Nov;30:310–316. doi: 10.1159/000208138. [DOI] [PubMed] [Google Scholar]
- Rej R., Richards A. H. Interference by Tris buffer in the estimation of protein by the Lowry procedure. Anal Biochem. 1974 Nov;62(1):240–247. doi: 10.1016/0003-2697(74)90383-2. [DOI] [PubMed] [Google Scholar]
- SPECTOR W. G., WILLOUGHBY D. A. The inflammatory response. Bacteriol Rev. 1963 Jun;27:117–154. doi: 10.1128/br.27.2.117-154.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weyden M. B., Buckley R. H., Kelley W. N. Molecular form of adenosine deaminase in severe combined immunodeficiency. Biochem Biophys Res Commun. 1974 Apr 8;57(3):590–595. doi: 10.1016/0006-291x(74)90587-7. [DOI] [PubMed] [Google Scholar]
- Wolberg G., Zimmerman T. P., Hiemstra K., Winston M., Chu L. C. Adenosine inhibition of lymphocyte-mediated cytolysis: possible role of cyclic adenosine monophosphate. Science. 1975 Mar 14;187(4180):957–959. doi: 10.1126/science.167434. [DOI] [PubMed] [Google Scholar]