Abstract
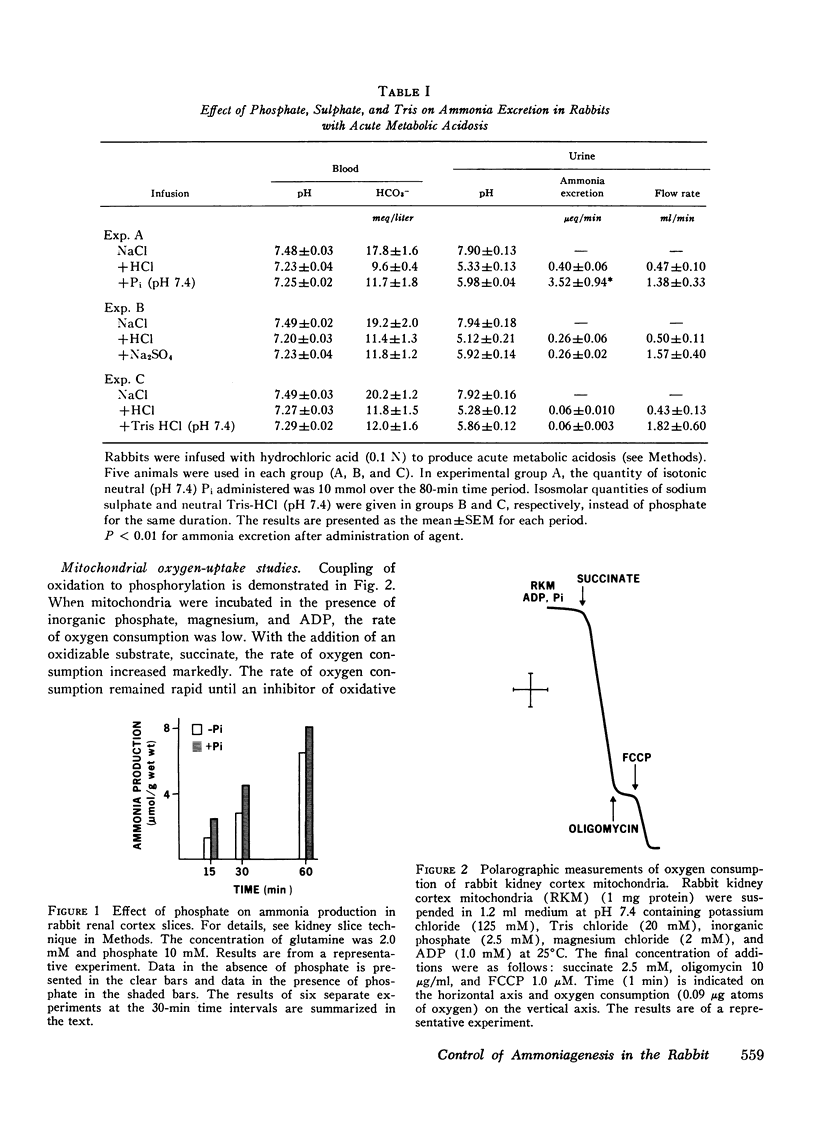
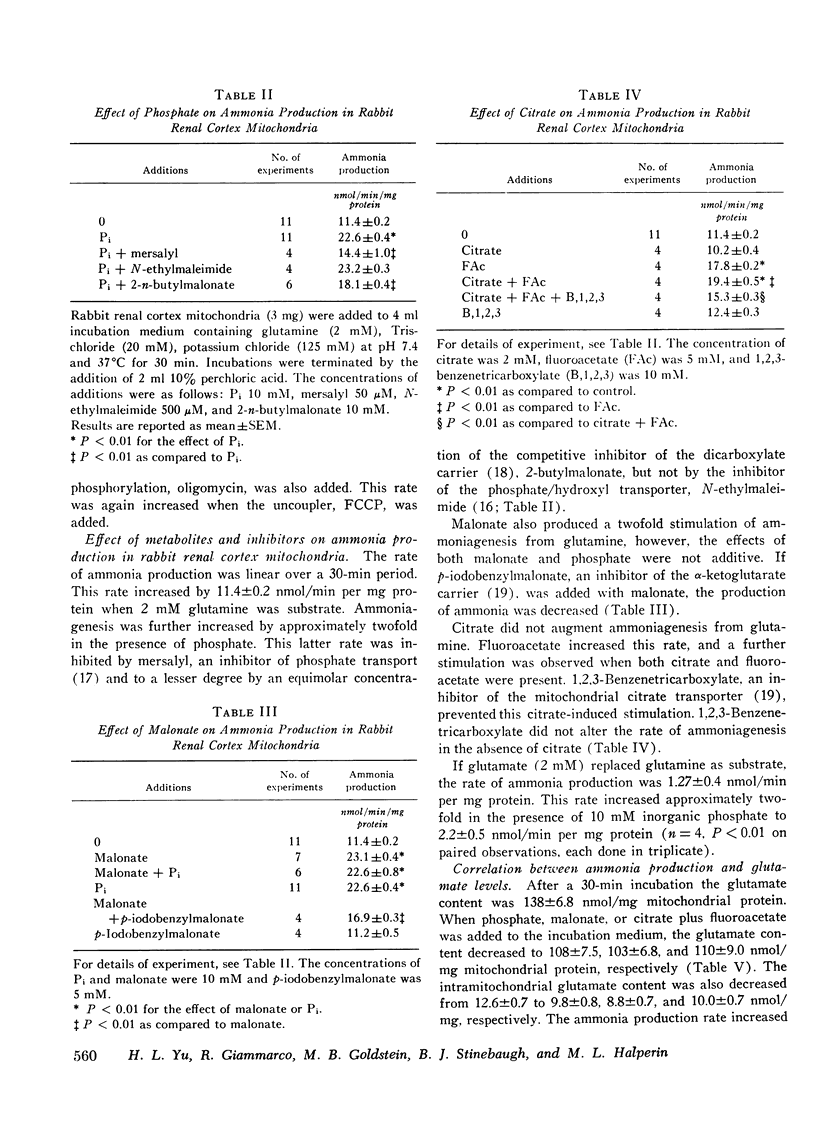
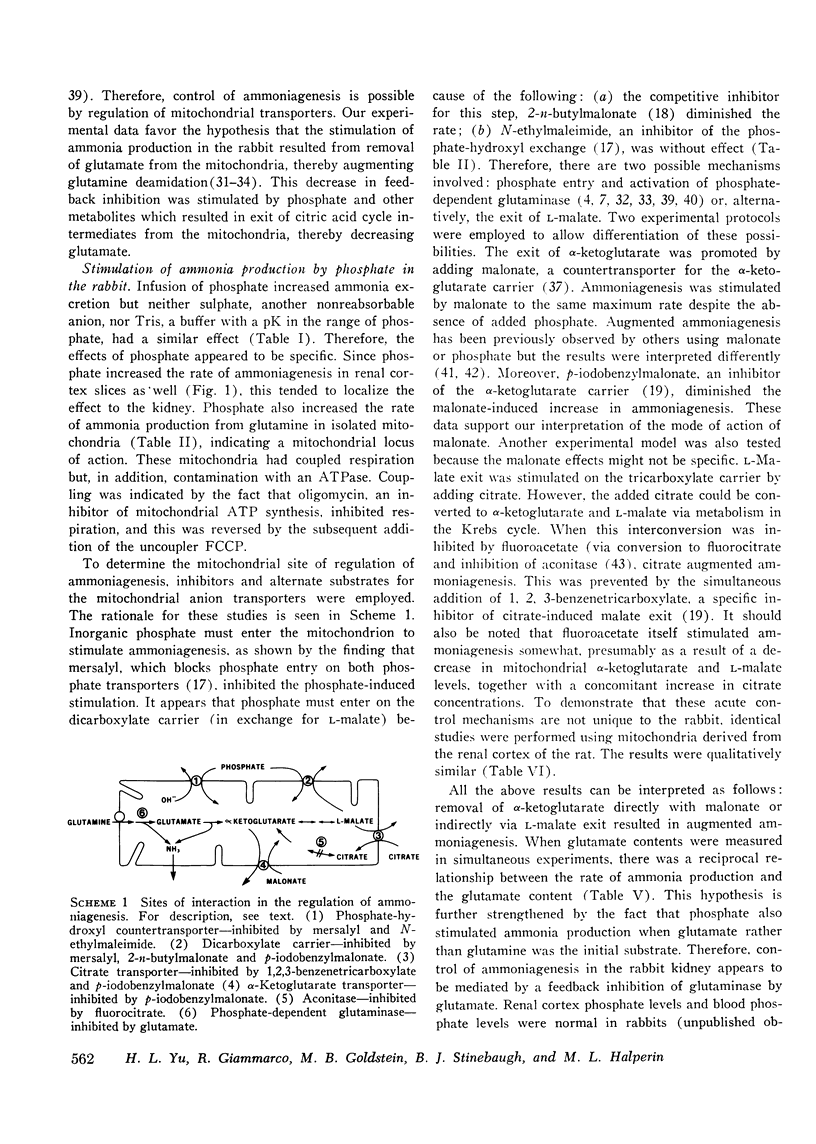
The purpose of this study was to clarify the mechanism (s) responsible for regulation of ammonia production and excretion in the rabbit. The normally low ammonia excretion rate during acute metabolic acidosis was stimulated acutely and increased approximately ninefold after infusion of sodium phosphate, but remained low if sodium sulphate or Tris was substituted for phosphate. Ammonia production was increased significantly by phosphate in rabbit renal cortex slices and in isolated renal cortex mitochondria. In isolated mitochondria, mersalyl, an inhibitor of both the phosphate/hydroxyl and phosphate/dicarboxylate mitochondrial carriers, inhibited the phosphate-induced stimulation, indicating that phosphate must enter the mitochondrion for stimulation. A malate/phosphate exchange seemed to be involved since N-ethylmaleimide, an inhibitor of the phosphate/hydroxyl exchange, did not inhibit phosphate-stimulated ammonia production, whereas there was inhibition by 2-n-butylmalonate, a competitive inhibitor of the dicarboxylate carrier. Phosphate itself was not essential since malonate stimulated ammoniagenesis in the absence of added phosphate. Similarly, citrate stimulated ammoniagenesis in isolated mitochondria in the absence of inorganic phosphate provided that it induced L-malate exit on the citrate transporter associated with inhibition of citrate oxidation by fluoroacetate. Similar results were also seen in mitochondria from rat renal cortex. A fall in mitochondrial alpha-ketoglutarate level resulted in an increase in ammonia production. This could be achieved directly with malonate or indirectly via L-malate exit. Simultaneous measurements of glutamate showed that the rate of ammonia production was reciprocally related to the glutamate content. We conclude that phosphate-induced stimulation of ammoniagenesis in the rabbit kidney is mediated by removal of glutamate, the feedback inhibitor of phosphate-dependent glutaminase. Glutamate removal is linked to phosphate-induced dicarboxylate exit across the mitochondrial membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W., Simpson D. P. Glutamine transport in rat kidney mitochondria in metabolic acidosis. J Clin Invest. 1974 Jul;54(1):165–174. doi: 10.1172/JCI107738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Hird F. J., Jones I. K. Metabolism of glutamine and ammonia in rat liver: the effects of N-acetylglutamate and phosphate. Arch Biochem Biophys. 1972 Sep;152(1):265–271. doi: 10.1016/0003-9861(72)90214-7. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the dog. Am J Physiol. 1970 Jan;218(1):241–245. doi: 10.1152/ajplegacy.1970.218.1.241. [DOI] [PubMed] [Google Scholar]
- Cunarro J. A., Weiner M. W. A comparison of methods for measuring urinary ammonium. Kidney Int. 1974 Apr;5(4):303–305. doi: 10.1038/ki.1974.41. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. Glutamate and glutamine distribution in the rat nephron in acidosis and alkalosis. Am J Physiol. 1973 Apr;224(4):884–889. doi: 10.1152/ajplegacy.1973.224.4.884. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN L., KENSLER C. J. Factors which affect the activity of glutaminase I in the guinea pig kidney. J Biol Chem. 1960 Apr;235:1086–1089. [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of metabolic acidosis and starvation on the content of intermediary metabolites in rat kidney. Biochem J. 1971 Jul;123(3):391–397. doi: 10.1042/bj1230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORGENSEN K. Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest. 1957;9(3):287–291. doi: 10.3109/00365515709079972. [DOI] [PubMed] [Google Scholar]
- Johnson R. N., Chappell J. B. The transport of inorganic phosphate by the mitochondrial dicarboxylate carrier. Biochem J. 1973 Jul;134(3):769–774. doi: 10.1042/bj1340769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra J., Brosnan J. T. The subcellular localization of glutaminase isoenzymes in rat kidney cortex. J Biol Chem. 1974 May 25;249(10):3255–3260. [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PETERS R. A. Further experiments on the inhibition of aconitase by enzymically prepared fluorocitric acid. Biochem J. 1961 May;79:261–268. doi: 10.1042/bj0790261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Preuss H. G. Renal glutamate metabolism in acute metabolic acidosis. Nephron. 1969;6(3):235–246. doi: 10.1159/000179731. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Vivatsi-Manos O., Vertuno L. L. Effects of glutamine deamination on glutamine deamidation in rat kidney slices. J Clin Invest. 1973 Apr;52(4):755–764. doi: 10.1172/JCI107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTERICH R. W., GOLDSTEIN L. Distribution of glutamine metabolizing enzymes and production of urinary ammonia in the mammalian kidney. Am J Physiol. 1958 Nov;195(2):316–320. doi: 10.1152/ajplegacy.1958.195.2.316. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. The effects of 2-ethylcitrate and tricarballylate on citrate transport in rat liver mitochondria and fatty acid synthesis in rat white adipose tissue. Eur J Biochem. 1970 Aug;15(2):263–272. doi: 10.1111/j.1432-1033.1970.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. The sensitivity of the exchange reactions of tricarboxylate, 2-oxoglutarate and dicarboxylate transporting systems of rat liver mitochondria to inhibition by 2-pentylmalonate, p-iodobenzylmalonate, and benzene 1,2,3-tricarboxylate. Eur J Biochem. 1971 May 11;20(1):65–71. doi: 10.1111/j.1432-1033.1971.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Simpson D. P., Adam W. Glutamine transport and metabolism by mitochondria from dog renal cortex. General properties and response to acidosis and alkalosis. J Biol Chem. 1975 Oct 25;250(20):8148–8158. [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]


