Abstract
The identification of genes underlying monogenic, early-onset forms of deafness in humans has provided unprecedented insight into the molecular mechanisms of hearing in the peripheral auditory system. The molecules involved in the development and function of the cochlea eluded characterization until recently due to the paucity of the principle cell types present in cochlear hair cells, yet a genetic approach has circumvented this problem and succeeded in identifying proteins and deciphering some of the molecular complexes that operate in these cells . In combination with mouse models, the genetic approach is now revealing some of the principles underlying the development and physiology of the cochlea. The review centers on this facet of the genetics of hearing. Focusing on the hair bundle, the mechanosensory device of the sensory hair cell, we highlight recent advances in understanding the way in which the hair bundle is formed, how it operates as a mechanotransducer and how it processes sound. In particular, we discuss how this work highlights the roles played by various hair-bundle link types.
Introduction
The two main roles of the human auditory system, analysis of the auditory scene1 and speech processing2, are both critically dependant upon the function of the sensory end organ, the cochlea. This organ transduces acoustic energy into electrical signals that are transmitted to the central auditory system via the auditory nerves. It also performs a spectral analysis of complex sounds and amplifies weak stimuli. In addition, it generates waveform distortions and allows some sounds to mask others, features essential for speech intelligibility and hearing in noisy environments. The sensory cells of the cochlea, the inner and outer hair cells (Fig. 1a, b) are the cells responsible for converting acoustic energy into electrical receptor potentials. The inner hair cells (IHCs) have a purely sensory role and transmit information to the brain, whilst the outer hair cells (OHCs) are sensori-motor cells that amplify low-level stimuli, and contribute to both frequency tuning and the distortion and masking of sounds.
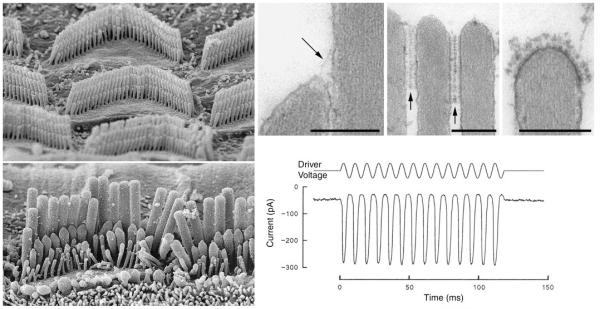
Figure 1. Hair bundle: Structure and Function.
(a, b) Scanning electron micrographs of the hair bundles of OHCs (a) and IHCs (b) in the adult mouse cochlea. (c-e) Transmission electron micrographs of a tip link (arrow, c), horizontal top connectors (arrows, d) and a tectorial membrane attachment crown (e) from mouse OHCs. (f) Transducer currents (bottom trace) recorded from a mouse cochlear OHC in response to sinusoidal stimulation of the hair bundle with a piezo-electric driven fluid jet (upper trace; voltage to piezo driver). Current flows into the cells (downward deflection in lower trace) when the hair bundle is deflected in the direction of the tallest row of stereocilia. Currents plotted include contribution from inward holding current. Micrographs in a and b were kindly provided by Prof. A. Forge, UCL, images in c-e are from Goodyear et al16, data in f are from Kros et al100. Scale bars in c-e = 200 nm.
The basic principles underlying the function of the ear, and especially of the cochlea, were mostly defined by physicists, with some of the earliest studies being initiated in the middle of the 19th century3-5. In contrast, the identification and characterization of the molecules involved in the development and function of the cochlea had to wait until the early 1990’s, mainly due to the paucity of the principle cell types present in this organ. The genetic approach has allowed this issue to be resolved6. Most recent reviews have focused on the molecular information derived from the study of hereditary deafness in humans and mice7-9. In addition to this top-down approach, genetics also offers the possibility of bottom-up approaches which advance our understanding of the basic principles underlying the development and physiology of tissues, organs and systems, and their interfaces with the environment.
However, addressing cochlear physiology through a genetic approach may not be straightforward. Many of the genes involved in cochlear physiology also play a critical role in the early differentiation of cochlear cells, and this early phenotype may hinder the detection of a functional role at later stages. The encoded proteins may underlie several functions that are not easy to disentangle. Compensatory mechanisms may develop in mutants that complicate interpretation. The primary functional defect may induce a cascade of secondary events that causes phenotypic anomalies that are only distantly related in space and time to the initial event. In some cases, for example in mutants with defects in the tectorial membrane, these issues do not arise and the knock-out/knock-in approach can be highly informative10. Otherwise conditional knockouts, conditionally expressed transgenes, or a genetic approach that allows one to turn off or modulate the activity of a protein and thus monitor the immediate effect (the ‘chemical-genetic’ strategy), are particular valuable tools for gaining insight into cochlear physiology.
The successful recent transfer of powerful biophysical and physiological technologies to the mouse ear has allowed the multidisciplinary analysis of mouse models, and thereby launched the physiological genetics of hearing. In this paper we focus on how the genetic approach has contributed to our recent understanding of the way in which the hair bundle, the mechanosensory structure of the hair cell, develops and functions.
Structure and function of hair bundles
Hair bundles are composed of 3-4 height-ranked rows of stereocilia: modified microvilli packed with highly cross-linked actin filaments (Fig. 1 a-d). OHC hair bundles have a distinct V or W-shape, while IHC hair bundles have a flatter, slightly curved profile (Fig. 1a, b). Two link types interconnect adjacent stereocilia in the mammalian cochlea: the oblique tip link (Fig. 1c) and the horizontal top connectors (Fig. 1d). The tip link (Fig. 1c) interconnects the stereocilia of adjacent rows, running from the tip of one stereocilium to the side of an adjacent taller stereocilium. It is oriented along the hair bundle’s axis of mechanosensitivity, and is thought to gate the hair cell’s mechanotransducer channel11,12. The top connectors (Fig. 1d) are multiple links that couple stereocilia both within and between the rows; they are distributed to varying extents along the upper regions of adjacent stereocilia. (In OHCs they form distinct, zipper-like junctions between the stereocilia 12-16. The tectorial membrane attachment crown (Fig. 1e) is an additional membrane specialisation present at the very tip of the tallest stereocilia in the OHC’s hair bundle13,14,16.
There is considerable evidence the hair cell’s elusive mechanotransducer channels are located near the tip of the hair bundle17-19. Current flows into the cells when the hair bundle is deflected in the direction of the tallest row of stereocilia, whilst movements in the opposite direction closes the channels that are open at rest (Fig. 1f). The prevailing theory for mechano-electrical transduction (MET) states that tension in a gating springs controls the MET channel opening probability20. Evidence suggests that the tip link, or a more compliant link in series with the tip link, comprises the gating spring11,21. Myosin motors have been proposed to set the tension in the gating spring22. When a steady state stimulus is applied to the hair bundle, hair cells restore their sensitivity to small stimuli by an adaptive feedback mechanism. Adaptation may contribute to the frequency tuning of auditory hair cells and is associated with active hair-bundle movements22-25 that may serve to amplify mechanical inputs to the hair cell26-28. Both the resting open probability and adaptation kinetics depend on the Ca2+ concentration close to the MET channel’s pore20,29-32. Ca2+ may decrease the tension exerted by myosins on the MET channel33 or may directly promote channel reclosure32,34,35.
Development of hair bundles
Hair bundle development has been described in detail for the avian auditory organ, the basilar papilla36. Three phases of development can be distinguished. The hair bundle starts off with a single, centrally-positioned kinocilium,surrounded by a few, very short microvilli. The kinocilium then migrates to the side and the microvilli immediately adjacent to the kinocilium elongate, followed by those in rows located further away, generating the staircase pattern of height-ranked stereocilia (Phase 1). The newly-formed stereocilia then become thicker, and the rootlets that will eventually anchor the stereocilia into the cuticular plate form, the network of actin filaments that lies just beneath the hair bundle (Phase 2). Finally, the stereocilia all start to elongate again, with those in the shortest row then stopping first followed by those in the progressively taller rows (Phase 3). This last process exaggerates differences in height ranking between the rows of stereocilia. Two other processes occur during these three phases: First, an excess of nascent stereocilia is produced as the apical surface of the hair cell expands during development. These are subsequently eliminated, leaving only those that have been incorporated into the height-ranked rows of stereocilia adjacent to the kinocilium. Second, although the initial migration of the kinocilium to one side of the hair determines the polarity of the bundle, a secondary rotation of the hair bundle (or possibly of the entire hair cell), brings the hair bundles of neighboring cells into alignment with one another determining the final planar polarity of the hair bundle37. The extent to which the process of hair-bundle development in the cochlea is similar to that seen in the avian auditory papilla remains open to some debate. For example, it appears unlikely that the elongation and widening of stereocilia occur in temporally separate phases as in the chick 38.
Unravelling hair-bundle development with deafness genes
Genes that have been implicated in controlling the normal development and/or the maintenance of hair-bundle structure and function are listed in Table 1. Many of these are human deafness genes, i.e genes that when mutated cause deafness These genes encode a number of myosin motors, actin-binding and actin-cross-linking proteins, scaffolding proteins, and transmembrane proteins. The involvement of myosin motors and other actin-interacting proteins is not surprising considering the actin-based nature of the stereocilium. Myosin motors control the trafficking of hair-bundle proteins39,40.They may also drive conformational changes in their interacting proteins thus potentially masking/unmasking some of their binding sites, and they may maintain stereociliary membrane tension. These effects may therefore be Ca2+ regulated. Trafficking may in particular exert some control on actin polymerization and treadmilling within the hair bundle (see for a review7,41). Notably, the genetic approach has revealed the molecular nature and function of hair-bundle links, and the role played by the kinocilium in hair-bundle development.
Table. Deafness genes involved in primary hair-bundle defects.
| Gene | Protein | Forms of human deafness* | Mouse mutants |
|---|---|---|---|
| MYO1A | Myosin IA (motor protein) | DFNA48 | NAc |
| MYO1C | Myosin-1c (motor protein) | DFNAd | Myo1cY61G-33−/− |
| MYH9 | Myosin IIA (motor protein) | DFNA17 Fechtner syndrome |
Myh9+/−b,e |
| MYO3A | Myosin IIIA (motor protein) | DFNB30 | NAc |
| MYO6 | Myosin VI (motor protein) | DFNA22; DFNB37 cardiomyopathy syndromea |
Snell's waltzer (sv); Twist (Twt) |
| MYO7A | Myosin VIIA (motor protein) | DFNB2; DFNA11 Usher syndrome 1Ba |
Shaker-1 (sh1) Headbanger (hdb) |
| MYO15 | Myosin XV (motor protein) | DFNB3 | Shaker-2 (sh2) |
| ACTG1 | γ-actin (cytoskeletal protein) | DFNA20/26 | NAc |
| ESPN | Espin (actin-bundling protein) | DFNB36 DFNAid |
Jerker (je) |
| RDX | Radixin (actin-binding protein) | DFNB24 | Rdx −/−b |
| TRIOBP | TRIOBP (actin-binding protein) | DFNB28 | NAc |
| HDIA1 | Diaphanous-1 (forming-homologous cytoskeleton regulatory protein) |
DFNA1 | NAc |
| Clic5 | Clic5 (chloride intracellular channel) | NAc | jitterbug (jbg) |
| USH1C | Harmonin (PDZ domain-containing protein) | DFNB18; Usher syndrome 1Ca | Deaf circler (dfcr) Ush1c−/−b |
| SANS | Sans (cytoskeletal protein) | Usher syndrome 1Ga | Jackson shaker (js) |
| WHRN | Whirlin (PDZ domain-containing protein) | DFNB31; Usher syndrome 2Da | Whirler (wi) |
| CDH23 | Cadherin-23 (integral membrane adhesion protein) |
DFNB12; Usher syndrome 1Da | Waltzer (v) |
| PCDH15 | Protocadherin-15 (cell adhesion protein) | DFNB23; Usher syndrome 1Fa | Ames waltzer (av) |
| VLGR1 | VLGR1 (Very large G protein-coupled receptor) | Usher syndrome 2Ca |
Vlgr1/del7TM
Vlgr1 −/−b |
| USH2A | Usherin (transmembrane protein) | Usher syndrome 2Aa | Ush2a −/−b |
| Ptprq | Ptprq (transmembrane protein) | NAc | Ptprq−/− |
| STRC | Stereocilin (extracellular protein) | DFNB16 | Strc −/−b |
| PMCA2 | PMCA2 (Ca2+ pump, ATP2B2) | modifier of CDH23 | deafwaddler (dfw); Atp2b2−/− |
| USH3A | Clarin-1 (integral protein) | Usher syndrome 3Aa | NAc |
| TMHS | TMHS (tetraspan) | DFNB67 | Hurry-scurry (hscy) |
| TMC1 | TMC1 (transmembrane channel-like protein) | DFNB7/11; DFNA36 | Deafness (dn); Beethoven (bth) |
| Trpml3 | Trpml3 (TRP channel) | NAc | Varitint-waddler (Va) |
| BBS1 | BBS1 (ciliary protein) | Bardet-Biedl form 1 | Bbs1 −/−b |
| BBS4 | BBS4 (ciliary protein) | Bardet-Biedl form 4 | Bbs4 −/−b |
| BBS8 | BBS8 (ciliary protein) | Bardet-Biedl form 8 | Bbs8 −/−b |
| Ift88 | Polaris (ciliary protein) | PKD | Ift88 CKO/CKOb |
Isolated and/or syndromic forms of human deafness are indicated
Syndromic deafness
Mutant obtained by gene knockout
NA: not available
Subscript i denotes undefined locus number
Mutant not deaf at heterozygous state, lethal at homozygous state
Isolated deafness: DFNA: autosomal dominant mode of inheritance; DFNB: autosomal recessive; DFN: X chromosome-linked transmission
Syndromic deafness forms presented:
Usher syndrome: sensorineural deafness associated to retinitis pigmentosa leading to blindness
Stickler syndrome: sensorineural deafness associated to facial anomalies and ocular degeneration, +/− other features
Fechtner syndrome: giant platelets disorder
Bardet-Biedl syndrome: obesity, retinopathy, polydactyly, hypogonadism, renal anomalies
PKD: Polycystic kidney disease, renal cysts
Details and references in Petit et al, 200199, Frolenkov et al, 20047, Friedman et al, 20078, Brown et al, 20089, Leibovici et al, 200869 See also websites: <http://webh01.ua.ac.be/hhh/>, <http://www.ncbi.nlm.nih.gov/sites/entrez?db=omim&TabCmd=Limits>
Role of the kinocilium
Although early morphological studies suggested a key role for the kinocilium in hair-bundle development, it is only recently that its function as sensor for the establishment of planar polarity within the cochlear epithelium is becoming clear. Mutations in a large number of genes are now known to disrupt the planar polarity of hair bundles42-48. These are not strictly deafness genes as their mutant phenotypes are usually(due to neural tube closure defects) lethal before the onset of hearing. Many of these genes encode non-canonical Wnt/planar cell polarity (PCP) pathway proteins, and some are distributed in a remarkably asymmetrical fashion within the cochlea, localizing to hair cell-supporting cell boundaries at either the medial (Vangl2, Fz3, Fz6) or lateral (Dvl2) poles of the hair cell45,49,50. Whilst these findings indicate a key role for the non-canonical Wnt/PCP pathway in establishing the orientation of hair bundles in the cochlea, it is unclear how the potential signal (possibly a localized Wnt source) is detected and orients the hair bundle.
Hair bundles are mis-oriented in mice carrying mutations in orthologues of some of the genes (BBS1, 4 and 8) responsible for Bardet-Biedl syndrome, a pleiotropic disorder due to ciliary dysfunction (see Table 1), and this provides genetic evidence for the kinocilium playing a role in defining planar polarity51. More striking evidence for a role for the kinocilium in defining planar polarity within the organ of Corti comes from a mouse with a conditional mutation in the gene encoding the intraflagellar/intraciliary transport protein Ift8852. The early conditional inactivation of Ift88 in the inner ear leads to a loss or under-development of the kinociliary axoneme. Hair bundles with organised height-ranked rows of stereocilia still form but are either circular, or polarised but misoriented, despite the presence of correctly (i.e., asymmetrically) located PCP pathway proteins. The basal body of the kinocilium is, however, still present and its position correlates with the polarity of the hair bundle, i.e., the hair bundle is circular (non-polarized) when the basal body fails to migrate from its central position, but polarized (but not correctly oriented with respect to the other hair cells or the epithelium) when the basal body migrates to the edge of the cell. The directional migration of the kinocilium is therefore a downstream target of the core PCP proteins sufficient to establish planar polarity. Genetic interactions of Vangl2 with Bbs1, Bbs8 and Ift88 have also been documented51,52 providing further evidence of a dialogue between the Wnt/PCP pathway and the kinocilium.
Whilst these results establish a role for the kinocilium as a PCP sensor, it is not yet known how the small GTPases activated by the non-canonical Wnt/PCP pathway53 result in the transfer of signals from the junctional PCP proteins to the basal body. Changes in actin polymerization or microtubule organization may help drive the basal body from the centre of the hair cell’s apical surface to the periphery, as may motor proteins.
Hair-bundle links: roles in cohesion
The study of deafness genes has revealed the molecular nature of the hair-bundle links and the proteins potentially involved in their interaction with the actin core of the stereocilium. Except for the tip-link, hair-bundle links had no known functions, but now turn out to have an essential role both during morphogenesis and at the adult stage. The most immediately visible role for several of these links is the maintenance of hair-bundle cohesion.
During hair-bundle development, numerous lateral links interconnect the stereocilia together as well as the stereocilia to the kinocilium. These display a highly dynamic expression pattern from the early stages of hair-bundle development through to adulthood (Fig. 2a). These include the transient lateral links, the kinociliary links and the ankle links. Transient lateral links are distributed extensively over the entire surface of the emerging stereocilia hair bundle, but disappear during the first few days after birth in the mouse. Kinociliary links connect the kinocilium to the immediately adjacent stereocilia and remain until the kinocilium disappears, at around P9 in the mouse. Ankle links become apparent around the basal region of the hair bundle at P2 and persist for only a short while, disappearing by P9. A dense cell coat, that forms shaft connectors in vestibular and lower vertebrate hair bundles, is only a transitory feature of the developing OHC hair bundle. Distinct horizontal top connectors appear relatively late during development but persist into adulthood. They can be first distinguished around P9 on OHCs, concomitant with the loss of the ankle links. The exact time at which tip links first appear is hard to ascertain by morphological analysis. Multiple links are often observed at the tip of each stereocilium during the early stages of hair-bundle development. These links are not dissimilar in appearance to the transient lateral links, often projecting to many or all of the neighbouring stereocilia, and but unlike the tip link, they are not uniquely aligned along hair bundles eventual axis of mechanosensitivity54,55. The presence of a very small mechanotransduction current as early as P1 in rat OHCs, however, argues in favor of the presence of a functional tip-link at this stage56.
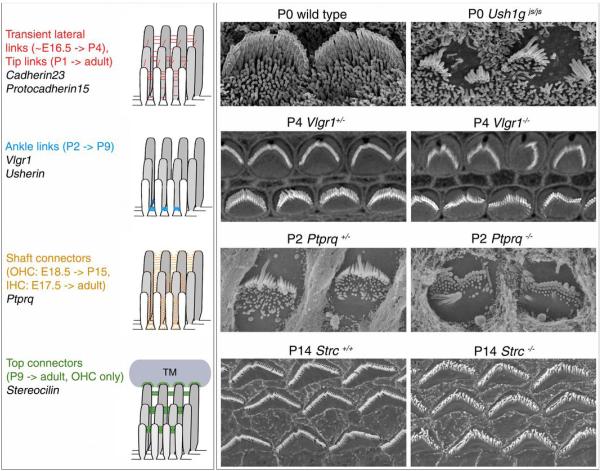
Figure 2. Hair-bundle development and hair-bundle phenotypes in mouse mutants.
a) Cartoon summarising the link types present at the different stages of development and their distribution in the hair bundle. b, c) Scanning electron micrographs of OHC hair bundles in wild type (b) and sans (Ush1gjs/js) mutant (c) mice at P0. Note how the hair bundles are fragmented in several small clumps of stereocilia in the mutant. d, e) Rhodamine-phalloidin stained hair bundles of IHCs (bottom row) and OHCs (top row) in P4 mice heterozygous (d) or homozygous (e) for a functional null mutation in Vlgr1. Note the loss of a sharply-defined hair bundle in the mutant. f, g) Scanning electron micrographs IHC hair bundles in P2 mice heterozygous (f) or homozygous (g) for a null mutation in the gene encoding Ptprq. Note how many of the stereocilia are shorter and how some are fused in the mutant. h, i) Scanning electron micrographs of P14 OHC hair bundles from wild type mice (h) or mice homozygous (g) for a null mutation in the gene encoding stereocilin. Note how the tips of the stereocilia are not well aligned in the mutant. Images in h and g are from Verpy et el.,93.
At least one component has been identified for each of the morphologically defined subsets of hair-bundle links.. Two members of the Ca2+ dependent cell-cell adhesion molecule superfamily, cadherin-23 and protocadherin-15, are components of the transient stereociliary lateral links, the kinociliary links, and the tip link57-63. The very large G-protein coupled receptor, Vlgr1, and usherin (also a very large transmembrane protein) form the ankle links40,64,65. The receptor-like inositol lipid phosphatase Ptprq is associated with the dense cell coat of auditory hair cells’ stereocilia and with the shaft connectors of vestibular hair cells16,66. The predicted extracellular protein stereocilin is associated with both the horizontal top connectors and the tectorial membrane attachment crowns of the OHCs67.
Available evidence suggests that the transient lateral links, the kinociliary links, the ankle links and the top connectors are all involved in hair-bundle cohesion. Cadherin-23 and protocadherin-15, along with myosin VIIa, the PDZ (post-synaptic density, discs large, zonula adherens) domain-containing protein harmonin, and the putative cytoskeletal protein sans, are all encoded by Usher type 1 syndrome genes, and mutations in any one of these genes lead to similar morphological hair-bundle anomalies. These include fragmentation of the hair bundle into several smaller clumps of stereocilia from the earliest stage of hair-bundle development onwards (Fig. 2b, c), along with a mis-positioned kinocilium frequently not associated with a stereocilia cluster68. These similarities, along with evidence for co-localisation, direct in vitro interactions, and the interdependence of localization in the hair bundle suggest these proteins operate in the same molecular complex (for review see Leibovici et al69). Harmonin, especially the b-isoform that can directly bind to F-actin39, most likely anchors the transient lateral stereocilia and kinociliary links to the actin core of the stereocilium, whereas myosin VIIa and sans ensure the correct localization of harmonin-b in the bundle39,68. In all the Usher type 1 mouse mutants, the kinocilium migrates from its central position to periphery, but fails to reach its final correct position. This suggests that the early cohesion of the hair bundle ensured by the transient lateral and kinociliary links also impinges the second migratory step of the kinocilium. It follows that the connection between the kinocilium and the stereocilia is likely to be already established by these links at this stage. The hair bundle may thus move as a single unit to a final position determined by the sum of the physical forces exerted on the entire hair bundle.
Vlgr1 and usherin are both encoded by the Usher syndrome type 2 genes. Mulitple protein modules (principally Calxb repeats for Vlrg1 and fibronectin type III repeats for Vlgr1) make up the large ectodomains of Vlgr1 and usherin. The intracellular domains of these two proteins both bind to the submembranous PDZ domain protein whirlin (the product of another Usher type 2 gene) and myosin VIIa 40. Together, these proteins form the ankle link molecular complex. In contrast to the situation with the Usher type 1 genes, a hair-bundle phenotype only becomes apparent in Vlgr1 mutant mice at postnatal day (P) 2 even though these proteins are produced from the very early stages of cochlear hair-bundle development. In this phenotype, the precise V-shape of the mutant hair bundle is no longer maintained (Fig. 2d, e)40,65. This suggests that the ankle-links missing in these mutant mice normally physically constraint the relationship between adjacent stereocilia40. Ptprq, a large transmembrane protein with an ectodomain containing multiple fibronectin III repeats, is associated with shaft connectors but is not thought to mediate cohesion of the bundle66. Ptprq mutants have shorter-than-normal stereocilia, but this phenotype is not visible until just after birth(Fig. 2f, g).
Finally, in stereocilin-deficient mice, hair bundle development is normal up until P1267. Horizontal top connectors are, however, absent in the hair bundles of the OHCs, and the precise alignment of stereocilia tips is lost by P14 (Fig. 2h, i). Horizontal top connectors therefore contribute to the proper cohesion and positioning of stereocilia tips with respect to one another in the mature hair bundle.
Thus, one subset of lateral links critically ensures hair-bundle cohesion at every stage (Fig. 2a). The transient lateral (stereociliary and kinociliary) links, the ankle links, and the horizontal top connectors appear sequentially in different locations - all over the hair bundle, around its base and at its apex .They are also composed of different classes of molecules. The type and position of the links are likely to be related to the direction and intensity of the forces applied to and/or generated within the hair bundle, and the degree of development of the stereociliary rootlets (ie, whether or not the stereocilia are anchored into the cuticular plate and additionally, as for the most peripheral stereocilia, to the plasma membrane at the cell junctions70.
One of the challenging issues is thus the determination of the varying forces applied to the hair bundle throughout its development and maturation. For example, the clusters of stereocilia that form in the absence of transient lateral links could be a consequence of the external forces resulting from the ongoing convergent-extension process driving cellular intercalation and cochlear elongation. On the other hand, one would expect the formation of stereocilia clusters of a characteristic size to be governed by a balance between the cohesive forces in the forming hair bundle, and forces driving the separation of stereocilia. In addition to these convergent-extension forces internal forces resulting from actin polymerisation within the stereocilia may play a role in maintaining this balance, as unconstrained growth would be likely to result in forces tending to splay stereocilia apart. With this view, the sequential reorganisation of stereocilia links and molecular motors during hair-bundle maturation is expected to shape its normal growth pattern by enabling the proper distribution of cohesive forces throughout the bundle. Myosin VIIa, a motor protein with a high duty ratio and a low ATPase activity71 associated both directly and indirectly with transient and ankle link components, has properties which would enable it to exert tension locally, and could participate in generating these forces72. Finally, additional, non-mechanical roles of these links have also be considered and have already been suggested for the predicted G-protein coupled receptor, Vlgr140.
Hair bundle links: roles in growth
Several deafness genes are known to cause defects in the growth of stereocilia:genes encoding myosin XVa73, whirlin74, espin75,76 and myosin VIIa68,77. Their roles have been the focus of several earlier reviews7,41,78, but it is still not known how they control actin polymerization in the stereocilia. We briefly focus on recent data that suggest a role of hair-bundle link proteins in stereocilia growth.
As mentioned above, the cochlear hair bundles of Ptprq−/− mice initially develop normally, but just after birth the stereocilia are shorter than normal and, in IHCs, they are sometimes enlarged and fused (Fig. 2f, g)66. As a receptor-like lipid phosphatase79, Ptprq has the potential to regulate the phosphatidyl inositol bi-phosphate content of the hair-bundle membrane and this, in turn, may impinge upon the activities of proteins that regulate actin turnover and stereocilia growth80. In all Usher type 1 mouse mutants, stereocilia growth is preserved up to P1 in the fragmented hair-bundle clumps, but in all except the Myo7a defective mutants, the middle and small rows fail to elongate between P1 and P568. This raises the intriguing possibility that the tip link, already present and functional at this date in mice54,81, controls the elongation of the stereocilia in the two shorter rows by the tension it exerts on the corresponding stereocilia tips63,68. Along the same line, Tilney and colleagues proposed some years ago that growth of the tallest row of stereocilia might activate the transducer channels in the shorter rows and therefore via, calcium influx, increase the actin polymerisation rate in the stereocilia of these rows82. Provided a mechanotransduction channel is located at the basal insertion point of the tip link, a coupling between maturation of the mechanotransduction machinery and the determination of stereocilia length is an appealing hypothesis.
Unraveling hair-bundle function via deafness genes
Mechanotransduction and adaptation
Currently several studies indicate that the Usher type 1 proteins, after their initial role in hair-bundle morphogenesis, form the core of the mechanotransduction apparatus. Although the mechanotransduction channel remains to be identified, the genetic approach has succeeded in providing arguments in favour of hypotheses suggesting motor molecules are involved in the adaptation of transducer currents.
Usher-1 proteins that are likely to form the core of the mechanotransduction machinery include cadherin-23, protocadherin-15 and harmonin-b. Evidence from immunoelectron microscopy indicates cadherin-23 and protocadherin-15 are associated with the upper and lower parts of the tip link, respectively, in mature hair bundles. Furthermore, the two proteins have been shown to interact via their N-termini in vitro and can form structures that bear, in rotary-shadowed replicas, a considerable resemblance to tip links63. Immunofluorescence microscopy has also revealed that harmonin-b is present at the tip of stereocilia during the initial growth the hair bundle, but is thereafter and into adulthood located below the tip of the stereocilia of tallest and middle rows68. At the resolution of the light microscope this region corresponds to the site at which the upper end of the tip link terminates. Association of harmonin-b with the top end of the tip link is reinforced by its known interaction with the cytoplasmic domain of cadherin-23 as well as its absence from this specific site in mutants defective for cadherin-2368. A direct interaction of harmonin b with the channel will depend on whether the channel is located at either the top or the bottom end of the tip link, or at both ends. Once this issue has been resolved, certain interpretations regarding the mechanisms underlying some of the characteristic features of mechanotransduction may need to be re-evaluated. Moreover, this will provide critical information for further deciphering the molecular machinery of mechanotransduction.
Based mostly on mRNA analysis, the gene for each of these three proteins is predicted to encode a variety of protein isoforms, three for cadherin-23 and more than ten and twenty for harmonin and protocadherin-15, respectively62,83 (and unpublished data). It is therefore possible that different isoforms of cadherin-23 and protocadherin-15 form the early transient hair-bundle lateral links and the tip links, and that different isoforms of harmonin form their respective F-actin anchoring proteins. For protocadherin-15, isoforms from the CD2 subgroup are the most likely candidates for components of the transient lateral and kinociliary links, and isoforms from the CD3 subgroup form the tip link62. The situation may, however, be more complex than this.
The genetic approach was also expected to provide a route to the mechanotransduction channel itself, unless a defect in this channel leads to embryonic lethality. Genetics has thus far identified a single hair-bundle cation channel, Trpml3, that is associated with severe, early-onset form of deafness84. Helix-breaking mutations in Trpml3 responsible for deafness result in constitutively active, inwardly-rectifying cation conductance85,86 that may cause calcium loading and thereby kill the hair cells87. Trpml3 is associated with the ankle links and, like the ankle links, is only transiently expressed in the developing hair bundle. The inwardly rectifying cation conductance is not blocked by blockers of the MET channel, suggesting Trpml3 is not involved in mechanotransduction87. Whilst alternative approaches are currently being developed for identifying the mechanotransduction channel, at least half the genes responsible for congenital deafness still remain to be identified, including some of the genes responsible for Usher type 1 syndrome. Furthermore it is worth noting that the identification of vertebrate mechanosensitive channels is, in general, a challenging issue. Only a few are known that are involved in the detection of mechanical stimuli. These include Pkd1/Pkd2, Trpa1, Trpc5, Trpv4 and Trpc1, but only the latter, Trpc1, has been shown to respond directly to mechanical stimulation and then only when expressed in oocytes88,89 .
Whilst these studies provide strong evidence that Usher proteins are part of the MET apparatus, functional evidence that cadherin-23 and protocadherin-15 form the tip link is thus far lacking, as is the characterization of the role and function of harmonin, especially harmonin-b, in the transduction process. Conditional knockouts that circumvent early hair-bundle defects or the knock-in of specific isoforms are required to assess the exact roles these proteins play in mechanotransduction. The functions of the other Usher type 1 proteins, myosin VIIa and sans, in mechanotransduction also remain to be evaluated. Using Usher proteins as entry points into the mechanotransduction machinery, a rapidly growing number of associated components can be anticipated (from yeast-two-hybrid screening combined with data emerging from analysis of the hair-bundle proteome90). These data should, in particular, allow one to assess the molecular identity of the gating spring. The Ca2+ modulated activity, folding, or molecular interactions of components of the mechanotransduction machinery should also rapidly become apparent.
Myosin-1c has long been considered as a primary candidate for the hair cell’s adaptation motor, and its role in this process has been explored in transgenic mice in which the ATP pocket in the motor head region has been enlarged91 to allow binding of the modified analogue of ADP, N6 (2-methyl butyl)-ADP (NMB-ADP). The activity of the modified, Y61G, myosin-1c can therefore be specifically inhibited by perfusing the hair cell with NMB-ADP during whole-cell patch current recording. This ‘chemical-genetic’ strategy has provided evidence that myosin-1c may be required for both the fast and slow components of transducer current adaptation, at least in mouse vestibular hair cells91,92. Because the kinetics of fast adaptation in auditory hair cells is too rapid to be dependent on the ATPase activity of myosin-1c, NMB-ADP has been proposed to relax myosin-1c92 so as to release tension and pre-adapt the system. Whilst these studies have provided strong evidence of a role for myosin-1c in adaptation, it should be noted that fast adaptation rates in auditory hair cells are considerably faster than those in vestibular hair cells. It will therefore be interesting to see how NMB-ADP affects adaptation in the cochlear hair cells of the Y61G myosin-1c mice. During cochlear development in the rat, the onset and maturation of adaptation precedes the main rise in myosin Ic immunoreactivity suggesting that there is an overabundance of myosin-1c the mature cochlear hair bundle relative to that required for adaptation or that myosin-1c may play additional roles56. Furthermore, increases in the rate of “slipping” adaptation and a loss of “climbing” adaptation have been reported for cochlear hair cells from the shaker-1 mice with an effective null mutation in the myosin VIIa gene81. An indirect effect on adaptation resulting from a reduction in membrane tension may explain the observed changes in adaptation seen in shaker-1 mice81.
The hair bundle as a source of cochlear waveform distortion
The genetic approach also led to the identification of unsuspected roles for the top connectors in the mature OHC hair bundle.
Stereocilin (Strc) is associated with the OHC horizontal top connectors and the tectorial membrane attachment crowns67,93, and its predicted amino acid sequence suggests it may be a secreted protein. In Strc−/− mice, the hair bundles of OHCs appear to develop normally until ~P12. Horizontal top connectors, however, are absent. By P14, there is a loss of the precise alignment of stereocilia tips (see above, Fig. 2 h, i) but the tip links are still present. Strc−/− mice, at the onset of hearing, are physiologically unique67: cochlear amplification and frequency tuning are normal but there is a total absence of any form of cochlear waveform distortion, and distortion product otoacoustic emissions (DPOAEs) cannot be elicited, even at high stimulus levels Similarly, there is a complete lack of any distortion in the cochlear microphonic potential waveforms. Waveform distortions in the cochlea, DPOAEs and suppressive masking were formerly attributed to the Boltzmann-shaped non-linearity of the mechanotransduction channel. The lack of waveform distortion in Strc−/− mice would thus suggest that their mechanotransduction transfer function has become linear over the waveform distortion-free 30-100 dB SPL range. This suggestion is, however, inconsistent with the fact that Strc−/− mice have normal hearing thresholds, at least at early stages. An alternative explanation to cochlear waveform distortions has thus been proposed67, ascribing a prominent role to n non-linear hair-bundle stiffness related to the horizontal top connectors: either directly or indirectly, these connectors could influence the stereocilia bundles in such a way that their movements distort sound waveforms. Whilst a putative loss of hair-bundle attachment to the tectorial membrane might be responsible for the loss of distortion in Strc−/− mice, this is unlikely as DPOAEs can be recorded at high sound pressure levels from mice in which the tectorial membrane is no longer in contact with the organ of Corti94.
Further exploration of the function of stereocilin runs into the general difficulties presently encountered when investigating cochlear physiology in adult mice. Technological improvements are, indeed, now required. Whilst approaches have been devised that are applicable to certain species95,96, a method for investigating mechanotransduction at the whole-cell level in the mature mouse cochlea still remains to be developed.
Conclusion
The use of deafness genes as entry points for understanding the mechanisms and principles of hair-bundle development and function has revealed has revealed unexpected results, such as a role for OHC top connectors in the production of waveform distortions. One of the salient features of the examples highlighted here is the convergence of data suggesting physical forces and associated mechano-sensitive mechanisms may control key steps of the morphogenesis of the hair bundle, a structure that has itself evolved to act as a mechano-electrical transducer. Although not yet investigated, reciprocal interactions between the hair bundle and the hair cell-supporting cell junctions, both of which are submitted to physical forces, may contribute to the development of each structure. The variety of the different myosins involved in deafness, with the present list certainly not being exhaustive, offers a range of mechanosensors to cope with a spectrum of tensions due to their motor activity and the way it is regulated to different extents by various loads. Considering the repertoire of cellular activities controlled by mechanical forces, it is an emerging and attractive possibility that the forces applied to some of the hair-bundle lateral links or hair cell-supporting cell junctions may control the activity of associated ionic channels97, or ensure the mechanical triggering of the synthesis or nuclear targeting of transcription factors, as has been observed in Drosophila98. This defines the measurement of the various afore mentioned forces as the next challenging issue.
Acknowledgements
The authors’ research was supported by grants from European Commission FP6 Integrated Project EuroHear LSHG-CT-2004-512063 (CP & GR), Fondation Raymonde et Guy Strittmatter (CP), Usher FAUN Stiftung (CP), Ernst-Jung Stiftung für Medizin (CP), Louis-Jeantet for Medicine Foundation (CP), and the Wellcome Trust (GR). The authors would like to thank V. Michel and R. Goodyear for their help in preparing the figures, A. Forge for the scanning electron micrographs presented in Figure 1, J. Levilliers for help in the preparation of the document and J-P Hardelin for his helpful criticism of the manuscript.
References
- 1.Bregman AS. Auditory Scene Analysis: The Perceptual Organization of sound. MIT Press; Cambridge, Massachusetts: 1990. [Google Scholar]
- 2.Moore BC, Tyler LK, Marslen-Wilson W, editors. The perception of speech: from sound to meaning, 917-1122. Royal Soc Publ; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohm GS. Über die Definition des Tones, nebst daran geknüpfter Theorie der Sirene und ähnlicher tonbildender Vorrichtungen. Annalen der Physik und Chemie. 1843;59:513–565. [Google Scholar]
- 4.von Helmholtz H. Die Lehre von den Tonempfindungen als physiologische Grundlage fur die Theorie derMusik (On the Sensations of Tone as a Physiological Basis for the Theory of Music) F. Vieweg und Sohn; Braunschweig: 1862. [Google Scholar]
- 5.Gold T. Hearing. II. The physical basis of the action of the cochlea. Proc R Soc Lond B Biol Sci. 1948;135:492–498. [Google Scholar]
- 6.Petit C. Genes responsible for human hereditary deafness: symphony of a thousand. Nature Genet. 1996;14:385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- 7.Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet. 2004;5:489–498. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- 8.Friedman LM, Dror AA, Avraham KB. Mouse models to study inner ear development and hereditary hearing loss. Int J Dev Biol. 2007;51:609–631. doi: 10.1387/ijdb.072365lf. [DOI] [PubMed] [Google Scholar]
- 9.Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9:277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- 10.Richardson GP, Lukashkin AN, Russell IJ. The tectorial membrane: one slice of a complex cochlear sandwich. Curr Opin Otolaryngol Head Neck Surg. 2008;16:458–464. doi: 10.1097/MOO.0b013e32830e20c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 12.Furness DN, Hackney CM. Cross-links between stereocilia in the guinea pig cochlea. Hear Res. 1985;18:177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- 13.Tsuprun V, Santi P. Structure of outer hair cell stereocilia links in the chinchilla. J Neurocytol. 1998;27:517–528. doi: 10.1023/a:1006903926571. [DOI] [PubMed] [Google Scholar]
- 14.Tsuprun V, Santi P. Structure of outer hair cell stereocilia side and attachment links in the chinchilla cochlea. J Histochem Cytochem. 2002;50:493–502. doi: 10.1177/002215540205000406. [DOI] [PubMed] [Google Scholar]
- 15.Tsuprun V, Schachern PA, Cureoglu S, Paparella M. Structure of the stereocilia side links and morphology of auditory hair bundle in relation to noise exposure in the chinchilla. J Neurocytol. 2003;32:1117–1128. doi: 10.1023/B:NEUR.0000021906.08847.d2. [DOI] [PubMed] [Google Scholar]
- 16.Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- 17.Hudspeth AJ. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982;2:1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaramillo F, Hudspeth AJ. Localization of the hair cell’s transduction channels at the hair bundle’s top by iontophoretic application of a channel blocker. Neuron. 1991;7:409–420. doi: 10.1016/0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- 19.Lumpkin EA, Hudspeth AJ. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc Natl Acad Sci U S A. 1995;92:10297–10301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- 22.Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog’s saccular hair cell. Proc Natl Acad Sci U S A. 1987;84:3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci. 2000;20:7131–7142. doi: 10.1523/JNEUROSCI.20-19-07131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin P, Bozovic D, Choe Y, Hudspeth AJ. Spontaneous oscillation by hair bundles of the bullfrog’s sacculus. J Neurosci. 2003;23:4533–4548. doi: 10.1523/JNEUROSCI.23-11-04533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA. 1999;96:14306–14311. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P, Hudspeth AJ. Compressive nonlinearity in the hair bundle’s active response to mechanical stimulation. Proc Natl Acad Sci USA. 2001;98:14386–14391. doi: 10.1073/pnas.251530498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- 29.Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog’s sacculus. J Neurosci. 1987;7:2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989;86:2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford AC, Evans MG, Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol. 1991;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron. 2008;59:530–545. doi: 10.1016/j.neuron.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung EL, Corey DP. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys J. 2006;90:124–139. doi: 10.1529/biophysj.105.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beurg M, Nam JH, Crawford A, Fettiplace R. The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J. 2008;94:2639–2653. doi: 10.1529/biophysj.107.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol. 1992;8:257–274. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- 37.Cotanche DA, Corwin JT. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear Res. 1991;52:379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- 38.Kaltenbach JA, Falzarano PR, Simpson TH. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J Comp Neurol. 1994;350:187–198. doi: 10.1002/cne.903500204. [DOI] [PubMed] [Google Scholar]
- 39.Boeda B, et al. Myosin VIIa, harmonin, and cadherin 23, three Usher I gene products, cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalski N, et al. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27:6478–6488. doi: 10.1523/JNEUROSCI.0342-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol. 2008;19:502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtin JA, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 43.Montcouquiol M, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 44.Lu X, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian D, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etheridge SL, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 52.Jones C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 53.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furness DN, Richardson GP, Russell IJ. Stereociliary bundle morphology in organotypic cultures of the mouse cochlea. Hear Res. 1989;38:95–109. doi: 10.1016/0378-5955(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 55.Pickles JO, von Perger M, Rouse GW, Brix J. The development of links between stereocilia in hair cells of the chick basilar papilla. Hear Res. 1991;54:153–163. doi: 10.1016/0378-5955(91)90116-q. [DOI] [PubMed] [Google Scholar]
- 56.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27:13890–13902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodyear RJ, Richardson GP. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J Neurosci. 2003;23:4878–4887. doi: 10.1523/JNEUROSCI.23-12-04878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siemens J, et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 59.Sollner C, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 60.Lagziel A, et al. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev. Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Michel V, et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev Biol. 2005;280:281–294. doi: 10.1016/j.ydbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed ZM, et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 64.Adato A, et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14:3921–3932. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- 65.McGee J, et al. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J Neurosci. 2006;26:6543–6553. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodyear RJ, et al. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci. 2003;23:9208–9219. doi: 10.1523/JNEUROSCI.23-27-09208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verpy E, et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;456:255–258. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lefevre G, et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 69.Leibovici M, Safieddine S, Petit C. Mouse models of human hereditary deafness. Curr Top Dev Biol. 2008;84:385–429. doi: 10.1016/S0070-2153(08)00608-X. [DOI] [PubMed] [Google Scholar]
- 70.Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci. 2008;28:6342–6353. doi: 10.1523/JNEUROSCI.1154-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henn A, De La Cruz EM. Vertebrate myosin VIIb is a high duty ratio motor adapted for generating and maintaining tension. J Biol Chem. 2005;280:39665–39676. doi: 10.1074/jbc.M507667200. [DOI] [PubMed] [Google Scholar]
- 72.El-Amraoui A, Bahloul A, Petit C. Myosin VII. In: Coluccio LM, editor. Myosins: A Superfamily of Molecular Motors. Vol. 7. Springer; New York: 2008. pp. 353–373. [Google Scholar]
- 73.Wang A, et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- 74.Mburu P, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 75.Donaudy F, et al. Espin gene (ESPN) mutations associated with autosomal dominant hearing loss cause defects in microvillar elongation or organisation. J Med Genet. 2006;43:157–161. doi: 10.1136/jmg.2005.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naz S, et al. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J Med Genet. 2004;41:591–595. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prosser HM, Rzadzinska AK, Steel KP, Bradley A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol Cell Biol. 2008;28:1702–1712. doi: 10.1128/MCB.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51:597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- 79.Oganesian A, et al. Protein tyrosine phosphatase RQ is a phosphatidylinositol phosphatase that can regulate cell survival and proliferation. Proc Natl Acad Sci U S A. 2003;100:7563–7568. doi: 10.1073/pnas.1336511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 81.Kros CJ, et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- 82.Tilney LG, Tilney MS, Cotanche DA. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J Cell Biol. 1988;106:355–365. doi: 10.1083/jcb.106.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verpy E, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nature Genet. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- 84.Di Palma F, et al. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99:14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grimm C, et al. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagata K, et al. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105:353–358. doi: 10.1073/pnas.0707963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Aken AF, et al. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol. 2008;586:5403–5418. doi: 10.1113/jphysiol.2008.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 89.Gottlieb P, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 90.Shin JB, et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holt JR, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 92.Stauffer EA, et al. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verpy E, et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nature Genet. 2001;29:345–349. doi: 10.1038/ng726. [DOI] [PubMed] [Google Scholar]
- 94.Legan PK, et al. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 95.He DZ, Jia S, Dallos P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature. 2004;429:766–770. doi: 10.1038/nature02591. [DOI] [PubMed] [Google Scholar]
- 96.Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mederos y Schnitzler M, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. Embo J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 99.Petit C, Levilliers J, Hardelin J-P. Molecular genetics of hearing loss. Annu. Rev. Genet. 2001;35:589–646. doi: 10.1146/annurev.genet.35.102401.091224. [DOI] [PubMed] [Google Scholar]
- 100.Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc. Roy. Soc. Lond. B. 1992;249:185–193. doi: 10.1098/rspb.1992.0102. [DOI] [PubMed] [Google Scholar]