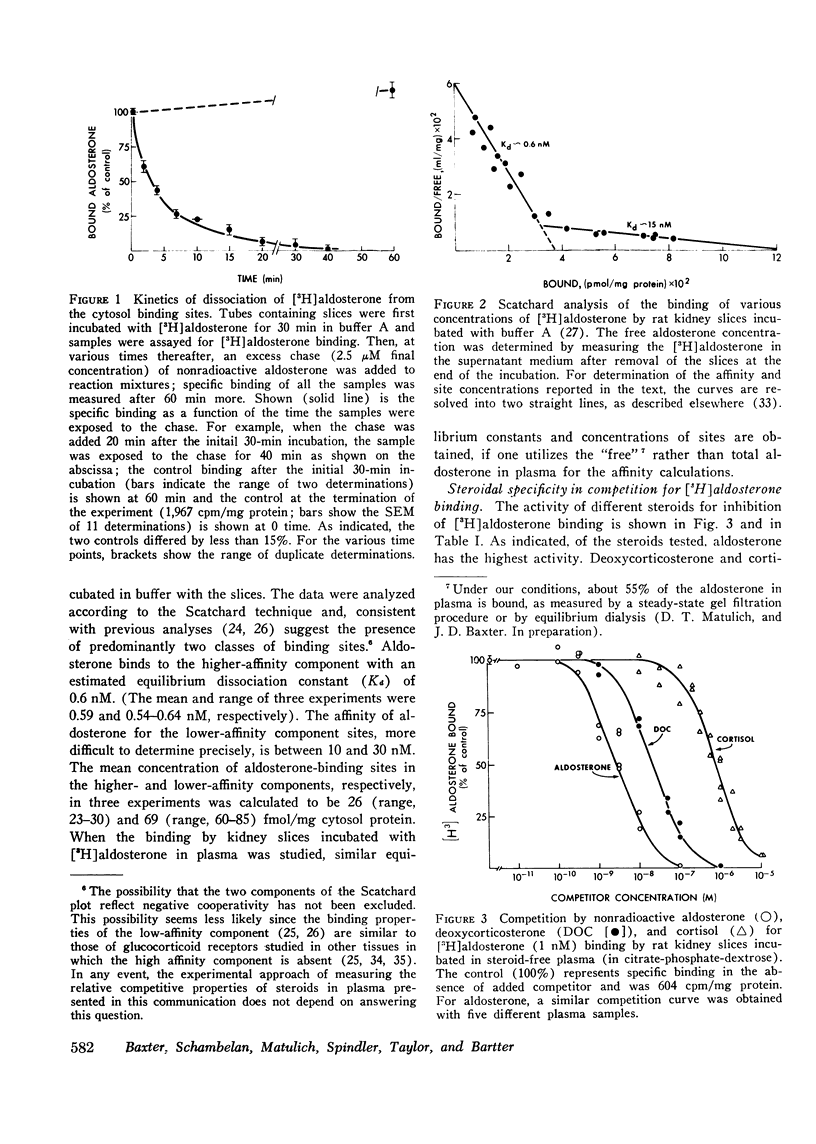
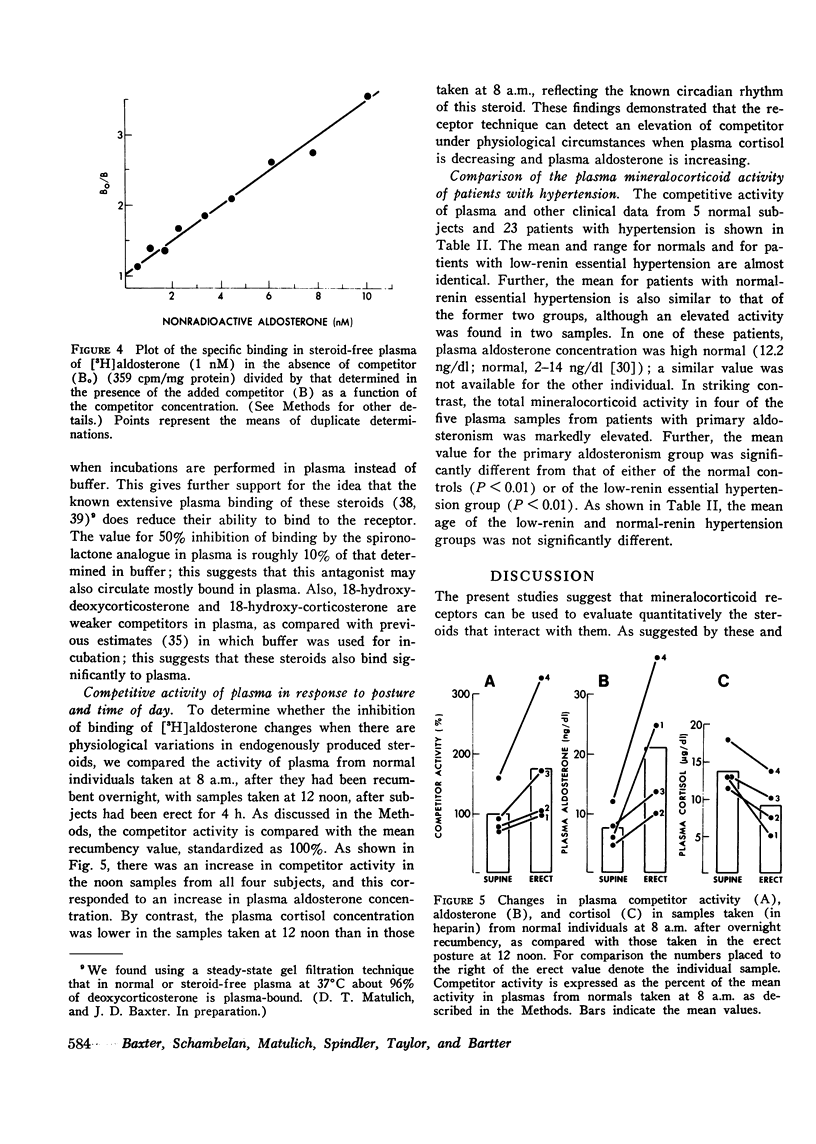
Abstract
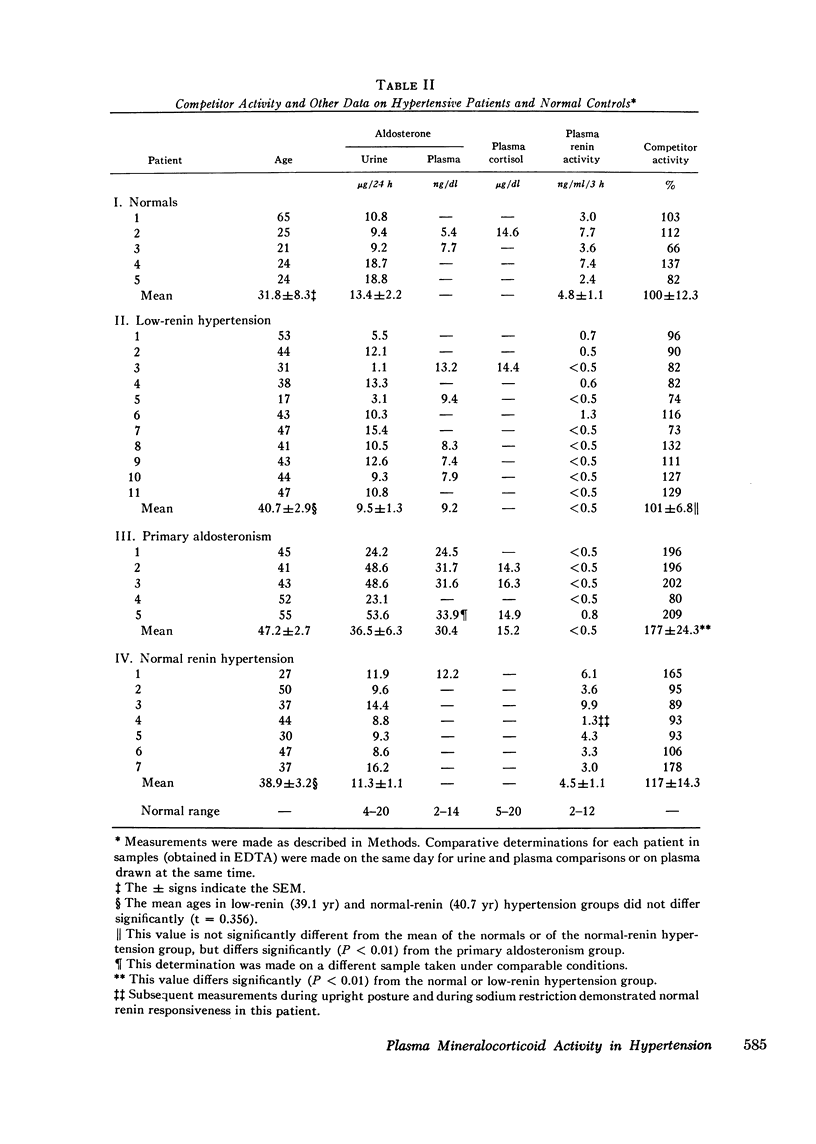
Aldosterone receptors from rat kidney slices were utilized in a competitive binding technique to analyze the contribution of various steroids to plasma "mineralocorticoid" activity and to assess their possible role in hypertension. To consider simultaneously the plasma binding, steroids were incubated with slices in undiluted plasma; competitor activities for [3H]aldosterone binding were aldosterone, 100%; deoxycorticosterone, 16.2%; cortisol, 0.4%; and 18-hydroxy-deoxy-corticosterone and d18-hydroxy-corticosterone, 0.1%. These steroids were more active in buffer than plasma, suggesting that they bind to plasma and that this reduces their receptor binding. Analysis of the competition data suggests that at normal plasma concentrations, aldosterone occupies the receptors to a major extent, cortisol occupies some of the receptors, and deoxycorticosterone and 8-hydroxydeoxycorticosterone contribute little to receptor occupancy. Two steroids implicated in low-renin essential hypertension, 16beta-hydroxy-dehydro-epiandrosterone and 16-oxoandrostenediol, did not have significant competitor activity. Competitor activity in plasmas from normal subjects taken at 12 noon (upright) was greater than that in those taken at 8 a.m. (supine). Since the 12 noon samples had higher aldosterone and lower cortisol levels than the 8 a.m. samples, the competitor activity under these physiological circumstances reflects aldosterone more than cortisol. The competitor activities of plasmas from patients relative to normal subjects (100+/-12.1%; mean+/-SEM) were: normal renin "essential" hypertension, 117+/-14%; low-renin essential hypertension, 101+/-6.6%; and primary aldosteronism, 176+/-14.3%. Thus a significant increase in activity of steroids that interact with mineralocorticoid receptors was detected in primary aldosteronism (P LESS THAN 0.01) BUT WAS NOT DETECTED IN LOW-RENIN OR NORMAL-RENIN ESSENTIAL HYPERTENSION.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlin E. V., Marks A. D., Channick B. J. Spironolactone and hydrochlorothiazide in essential hypertension. Blood pressure response and plasma renin activity. Arch Intern Med. 1972 Dec;130(6):855–858. [PubMed] [Google Scholar]
- BARTTER F. C., FOURMAN P. The different effects of aldosterone-like steroids and hydrocortisone-like steroids on urinary excretion of potassium and acid. Metabolism. 1962 Jan;11:6–20. [PubMed] [Google Scholar]
- BONGIOVANNI A. M., ROOT A. W. The adrenogenital syndrome. N Engl J Med. 1963 Jun 6;268:1283–contd. doi: 10.1056/NEJM196306062682308. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Carter J. P., Graham B. S., Baxter J. D. A radioreceptor assay for evaluation of the plasma glucocorticoid activity of natural and synthetic steroids in man. J Clin Endocrinol Metab. 1975 Aug;41(2):290–304. doi: 10.1210/jcem-41-2-290. [DOI] [PubMed] [Google Scholar]
- Biglieri E. G., Herron M. A., Brust N. 17-hydroxylation deficiency in man. J Clin Invest. 1966 Dec;45(12):1946–1954. doi: 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. J., Fraser R., Love D. R., Ferriss J. B., Lever A. F., Robertson J. I., Wilson A. Apparently isolated excess deoxycorticosterone in hypertension. A variant of the mineralocorticoid-excess syndrome. Lancet. 1972 Aug 5;2(7771):243–247. doi: 10.1016/s0140-6736(72)91685-6. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Strott C. A. Plasma deoxycorticosterone in man. J Clin Endocrinol Metab. 1971 Jun;32(6):744–750. doi: 10.1210/jcem-32-6-744. [DOI] [PubMed] [Google Scholar]
- Burton R. M., Westphal U. Steroid hormone-binding proteins in blood plasma. Metabolism. 1972 Mar;21(3):253–276. doi: 10.1016/0026-0495(72)90048-0. [DOI] [PubMed] [Google Scholar]
- CHEN P. S., Jr, MILLS I. H., BARTTER F. C. Ultrafiltration studies of steroid-protein binding. J Endocrinol. 1961 Oct;23:129–137. doi: 10.1677/joe.0.0230129. [DOI] [PubMed] [Google Scholar]
- Carey R. M., Douglas J. G., Schweikert J. R., Liddle G. W. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization of blood pressure with spironolactone. Arch Intern Med. 1972 Dec;130(6):849–854. [PubMed] [Google Scholar]
- Crane M. G., Harris J. J. Effect of spironolactone in hypertensive patients. Am J Med Sci. 1970 Dec;260(6):311–330. doi: 10.1097/00000441-197012000-00001. [DOI] [PubMed] [Google Scholar]
- Creditor M. C., Loschky U. K. Plasma renin activity in hypertension. Am J Med. 1967 Sep;43(3):371–382. doi: 10.1016/0002-9343(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Duval D., Funder J. W. The binding of tritiated aldosterone in the rat liver cytosol. Endocrinology. 1974 Feb;94(2):575–579. doi: 10.1210/endo-94-2-575. [DOI] [PubMed] [Google Scholar]
- Feldman D., Funder J. W., Edelman I. S. Subcellular mechanisms in the action of adrenal steroids. Am J Med. 1972 Nov;53(5):545–560. doi: 10.1016/0002-9343(72)90152-0. [DOI] [PubMed] [Google Scholar]
- Feldman D., Funder J. W. The binding of 18-hydroxydeoxycorticosterone and 18-hydroxycorticosterone to mineralocorticoid and glucocorticoid receptors in the rat kidney. Endocrinology. 1973 May;92(5):1389–1396. doi: 10.1210/endo-92-5-1389. [DOI] [PubMed] [Google Scholar]
- Fishman L. M., Küchel O., Liddle G. W., Michelakis A. M., Gordon R. D., Chick W. T. Incidence of primary aldosteronism uncomplicated "essential" hypertension. A prospective study with elevated aldosterone secretion and suppressed plasma renin activity used as diagnostic criteria. JAMA. 1968 Aug 12;205(7):497–502. doi: 10.1001/jama.205.7.497. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. Glucocorticoid receptors in rat kidney: the binding of tritiated-dexamethasone. Endocrinology. 1973 Apr;92(4):1005–1013. doi: 10.1210/endo-92-4-1005. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology. 1973 Apr;92(4):994–1004. doi: 10.1210/endo-92-4-994. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Highland E., Edelman I. S. Molecular modifications of anti-aldosterone compounds: effects on affinity of spirolactones for renal aldosterone receptors. Biochem Pharmacol. 1974 May 15;23(10):1493–1501. doi: 10.1016/0006-2952(74)90386-4. [DOI] [PubMed] [Google Scholar]
- LIDDLE G. W. Effects of anti-inflammatory steroids on electrolvte metabolism. Ann N Y Acad Sci. 1959 Oct 14;82:854–867. doi: 10.1111/j.1749-6632.1960.tb44967.x. [DOI] [PubMed] [Google Scholar]
- Lebel M., Schalekamp M. A., Beevers D. G., Brown J. J., Davies D. L., Fraser R., Kremer D., Lever A. F., Morton J. J., Robertson J. I. Sodium and the renin-angiotensin system in essential hypertension and mineralocorticoid excess. Lancet. 1974 Aug 10;2(7876):308–309. doi: 10.1016/s0140-6736(74)91690-0. [DOI] [PubMed] [Google Scholar]
- Marver D., Goodman D., Edelman I. S. Relationships between renal cytoplasmic and nuclear aldosterone-receptors. Kidney Int. 1972 Apr;1(4):210–223. doi: 10.1038/ki.1972.31. [DOI] [PubMed] [Google Scholar]
- Mason P. A., Fraser R. Estimation of aldosterone, 11-deoxycorticosterone, 18-hydroxy-11-deoxy-corticosterone, corticosterone, cortisol and 11-deoxycortisol in human plasma by gas-liquid chromatography with electron capture detection. J Endocrinol. 1975 Feb;64(2):277–288. doi: 10.1677/joe.0.0640277. [DOI] [PubMed] [Google Scholar]
- Melby J. C., Dale S. L. Adrenal steroidogenesis in "low renin" or hyporeninemic hypertension. J Steroid Biochem. 1975 May;6(5):761–766. doi: 10.1016/0022-4731(75)90065-5. [DOI] [PubMed] [Google Scholar]
- Melby J. C., Dale S. L., Wilson T. E. 18-Hydroxy-deoxycorticosterone in human hypertension. Circ Res. 1971 May;28(5 Suppl):143–152. doi: 10.1161/01.res.28.5.ii-143. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Oddie C. J., Coghlan J. P., Scoggins B. A. Plasma desoxycorticosterone levels in man with simultaneous measurement of aldosterone, corticosterone, cortisol and 11-deoxycortisol. J Clin Endocrinol Metab. 1972 Jun;34(6):1039–1054. doi: 10.1210/jcem-34-6-1039. [DOI] [PubMed] [Google Scholar]
- Oliver J. T., Birmingham M. K., Bartova A., Li M. P., Chan T. H. Hypertensive action of 18-hydroxydeoxycorticosterone. Science. 1973 Dec 21;182(4118):1249–1251. doi: 10.1126/science.182.4118.1249. [DOI] [PubMed] [Google Scholar]
- Powell-Jackson J. D., Calin A., Fraser R., Grahame R., Mason P., Missen G. A., Powell-Jackson P. R., Wilson A. Excess deoxycorticosterone secretion from adrenocortical carcinoma. Br Med J. 1974 Apr 6;2(5909):32–33. doi: 10.1136/bmj.2.5909.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp J. P., Dahl L. K. Possible role of 18-hydroxy-deoxycorticosterone in hypertension. Nature. 1972 Jun 9;237(5354):338–339. doi: 10.1038/237338a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Rousseau G., Baxter J. D., Funder J. W., Edelman I. S., Tomkins G. M. Glucocorticoid and mineralocorticoid receptors for aldosterone. J Steroid Biochem. 1972 Feb;3(2):219–227. doi: 10.1016/0022-4731(72)90053-2. [DOI] [PubMed] [Google Scholar]
- Schalekamp M. A., Lebel M., Beevers D. G., Fraser R., Kolsters G., Birkenhäger W. H. Body-fluid volume in low-renin hypertension. Lancet. 1974 Aug 10;2(7876):310–311. doi: 10.1016/s0140-6736(74)91691-2. [DOI] [PubMed] [Google Scholar]
- Schambelan M., Slaton P. E., Jr, Biglieri E. G. Mineralocorticoid production in hyperadrenocorticism. Role in pathogenesis of hypokalemic alkalosis. Am J Med. 1971 Sep;51(3):299–303. doi: 10.1016/0002-9343(71)90264-6. [DOI] [PubMed] [Google Scholar]
- Spark R. F., Melby J. C. Hypertension and low plasma renin activity: presumptive evidence for mineralocorticoid excess. Ann Intern Med. 1971 Dec;75(6):831–836. doi: 10.7326/0003-4819-75-6-831. [DOI] [PubMed] [Google Scholar]
- Spark R. F., O'Hare C. M., Regan R. M. Low-renin hypertension. Restoration of normotension and renin responsiveness. Arch Intern Med. 1974 Feb;133(2):205–211. doi: 10.1001/archinte.133.2.205. [DOI] [PubMed] [Google Scholar]
- Stockigt J. R., Collins R. D., Noakes C. A., Schambelan M., Biglieri E. G. Renal-vein renin in various forms of renal hypertension. Lancet. 1972 Jun 3;1(7762):1194–1197. doi: 10.1016/s0140-6736(72)90922-1. [DOI] [PubMed] [Google Scholar]
- Vaughan E. D., Jr, Laragh J. H., Gavras I., Bühler F. R., Gavras H., Brunner H. R., Baer L. Volume factor in low and normal renin essential hypertension. Treatment with either spironolactone or chlorthalidone. Am J Cardiol. 1973 Sep 20;32(4):523–532. doi: 10.1016/s0002-9149(73)80044-x. [DOI] [PubMed] [Google Scholar]
- Woods J. W., Liddle G. W., Michelakis A. M., Brill A. B. Effect of an adrenal inhibitor in hypertensive patients with suppressed renin. Arch Intern Med. 1969 Apr;123(4):366–370. [PubMed] [Google Scholar]


