Abstract
Bombesin (a tetradecapeptide), the C-terminal nonapeptide of bombesin (bombesin-NP), and litorin (a parent nonapeptide), each stimulated amylase secretion from rat pancreatic fragments. These responses were not affected by atropine. The concentrations that produced half-maximal stumulation of secretion were 0.25 nM for bombesin, 0.30 nM for bombesin-NP, and 0.07 nM for litorin, as compared to 0.12 nM for caerulein and 0.80 muM for the cholinergic agent carbamylcholine. When used at maximal concentrations, bombesin, bombesin-NP, and litorin showed no action on cyclic AMP levels in the presence of 5 mM theophylline. By contrast, caerulein and secretin increased cyclic AMP levels by 27 and 208%, respectively. Bombesin, bombesin-NP, and litorin did not activate adenylate cyclase in a purified pancreatic plasma membrane preparation, whereas caerulein and secretin increased this activity 20 and 16-times, respectively...
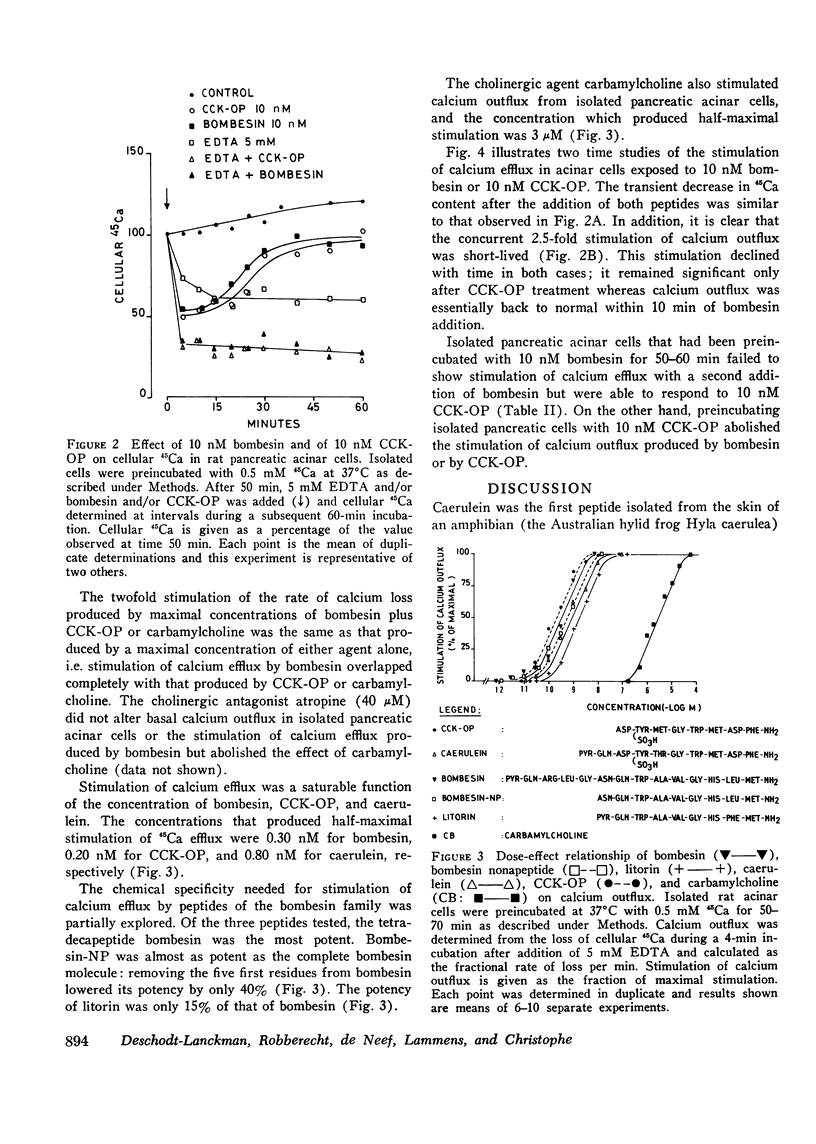
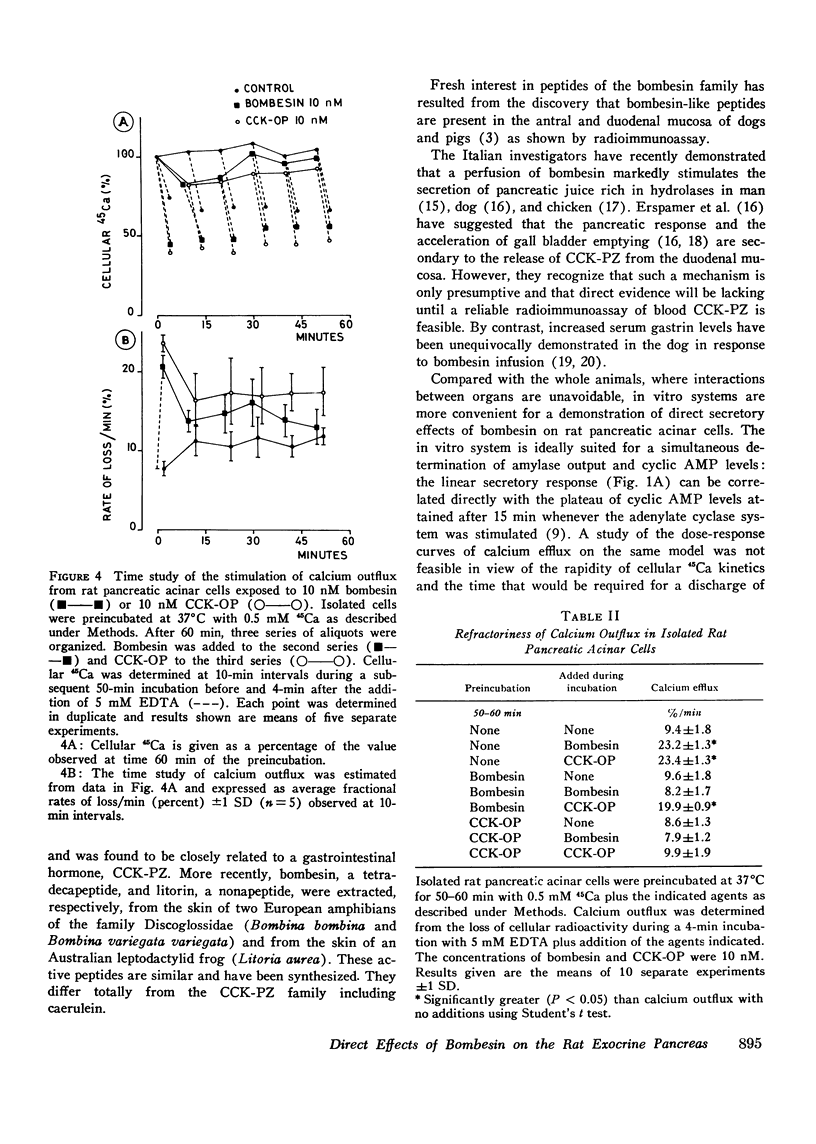
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini G., Erspamer V., Melchiorri P., Sopranzi N. Gastrin release by bombesin in the dog. Br J Pharmacol. 1974 Oct;52(2):219–225. doi: 10.1111/j.1476-5381.1974.tb09703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo M., Falconieri Erspamer G., Melchiorri P., Negri L., de Castiglione R. Relative potency of bombesin-like peptides. Br J Pharmacol. 1975 Oct;55(2):221–227. doi: 10.1111/j.1476-5381.1975.tb07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J., Robberecht P., Deschodt-Lanckman M., Lambert M., Van Leemput-Coutrez M., Camus J. Molecular basis of enzyme secretion by the exocrine pancreas. Adv Cytopharmacol. 1974;2:47–61. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Labrie F., Christophe J. In vitro interactions of gastrointestinal hormones on cyclic adenosine 3':5'-monophosphate levels and amylase output in the rat pancreas. Gastroenterology. 1975 Feb;68(2):318–325. [PubMed] [Google Scholar]
- Emås S., Billings A., Grossman M. I. Effects of gastrin and pentagastrin on gastric and pancreatic secretion in dogs. Scand J Gastroenterol. 1968;3(3):234–240. doi: 10.3109/00365526809180595. [DOI] [PubMed] [Google Scholar]
- Endean R., Erspamer V., Falconieri Erspamer G., Improta G., Melchiorri P., Negri L., Sopranzi N. Parallel bioassay of bombesin and litorin, a bombesin-like peptide from the skin of Litoria aurea. Br J Pharmacol. 1975 Oct;55(2):213–219. doi: 10.1111/j.1476-5381.1975.tb07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V., Improta G., Melchiorri P., Sopranzi N. Evidence of cholecystokinin release by bombesin in the dog. Br J Pharmacol. 1974 Oct;52(2):227–232. doi: 10.1111/j.1476-5381.1974.tb09704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Adams T. D. Cyclic AMP in pancreatic acinar cells: effects of gastrointestinal hormones. Gastroenterology. 1976 Jan;70(1):29–35. [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. I. Candidate hormones of the gut. I. Introduction. Gastroenterology. 1974 Oct;67(4):730–731. [PubMed] [Google Scholar]
- Grossman M. I. Letter: Additional candidate hormones of the gut. Gastroenterology. 1975 Aug;69(2):570–571. [PubMed] [Google Scholar]
- Haymovits A., Scheele G. A. Cellular cyclic nucleotides and enzyme secretion in the pancreatic acinar cell. Proc Natl Acad Sci U S A. 1976 Jan;73(1):156–160. doi: 10.1073/pnas.73.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Schulz I. Calcium ion uptake in isolated pancreas cells induced by secretagogues. Biochim Biophys Acta. 1976 Jan 8;419(1):76–92. doi: 10.1016/0005-2736(76)90373-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., De Neef P., Borgeat P., Christophe J. In vivo effects of pancreozymin, secretin, vasoactive intestinal polypeptide and pilocarpine on the levels of cyclic AMP and cyclic GMP in the rat pancreas. FEBS Lett. 1974 Jul 15;43(2):139–143. doi: 10.1016/0014-5793(74)80986-5. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., De Neef P., Christophe J. Effects of somatostatin on pancreatic exocrine function. Interaction with secretin. Biochem Biophys Res Commun. 1975 Nov 3;67(1):315–323. doi: 10.1016/0006-291x(75)90318-6. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur J Biochem. 1972 Jul 13;28(2):199–204. doi: 10.1111/j.1432-1033.1972.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Stening G. F., Grossman M. I. Gastrin-related peptides as stimulants of pancreatic and gastric secretion. Am J Physiol. 1969 Jul;217(1):262–266. doi: 10.1152/ajplegacy.1969.217.1.262. [DOI] [PubMed] [Google Scholar]
- Vandermeers A., Vandermeers-Piret M. C., Christophe J. Kinetics of soluble starch hydrolysis by rat and human -amylases and its bearing on automated single-time point saccharogenic assays. Biochimie. 1971;53(8):859–864. doi: 10.1016/s0300-9084(71)80148-7. [DOI] [PubMed] [Google Scholar]


