Abstract
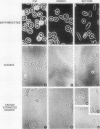
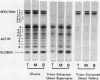
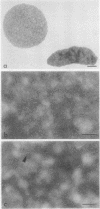
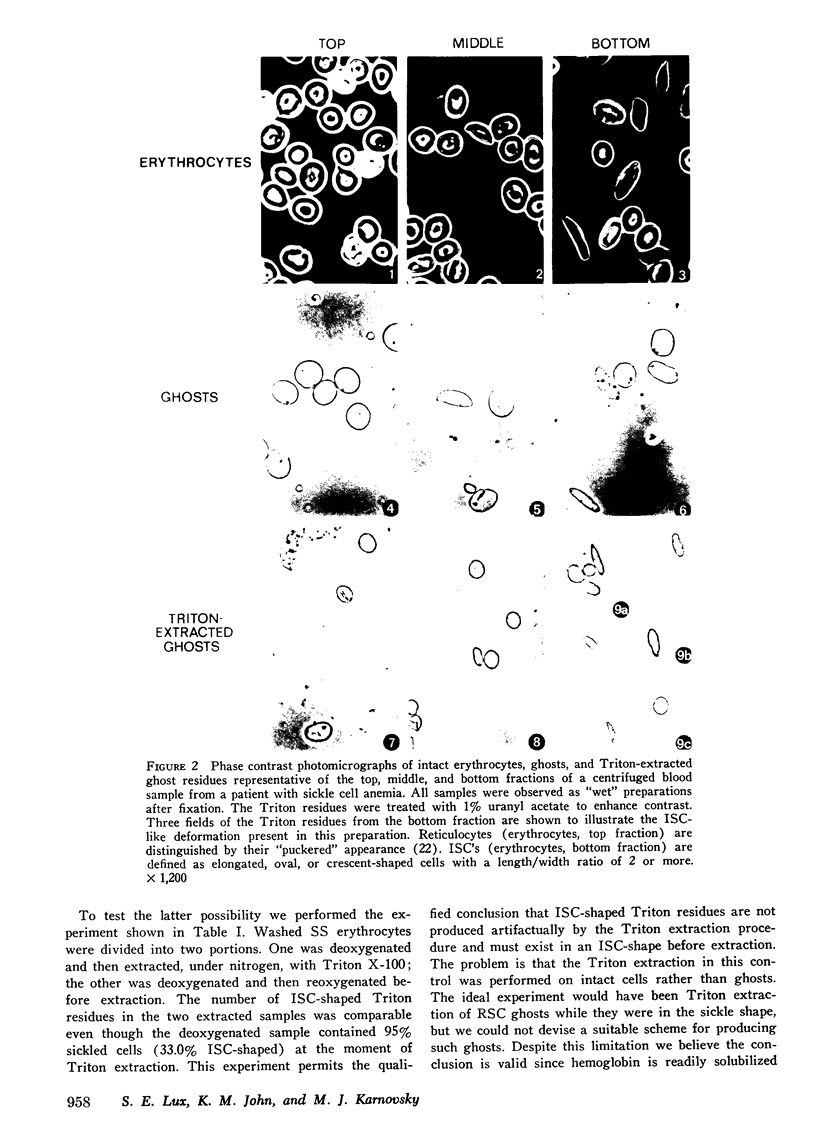
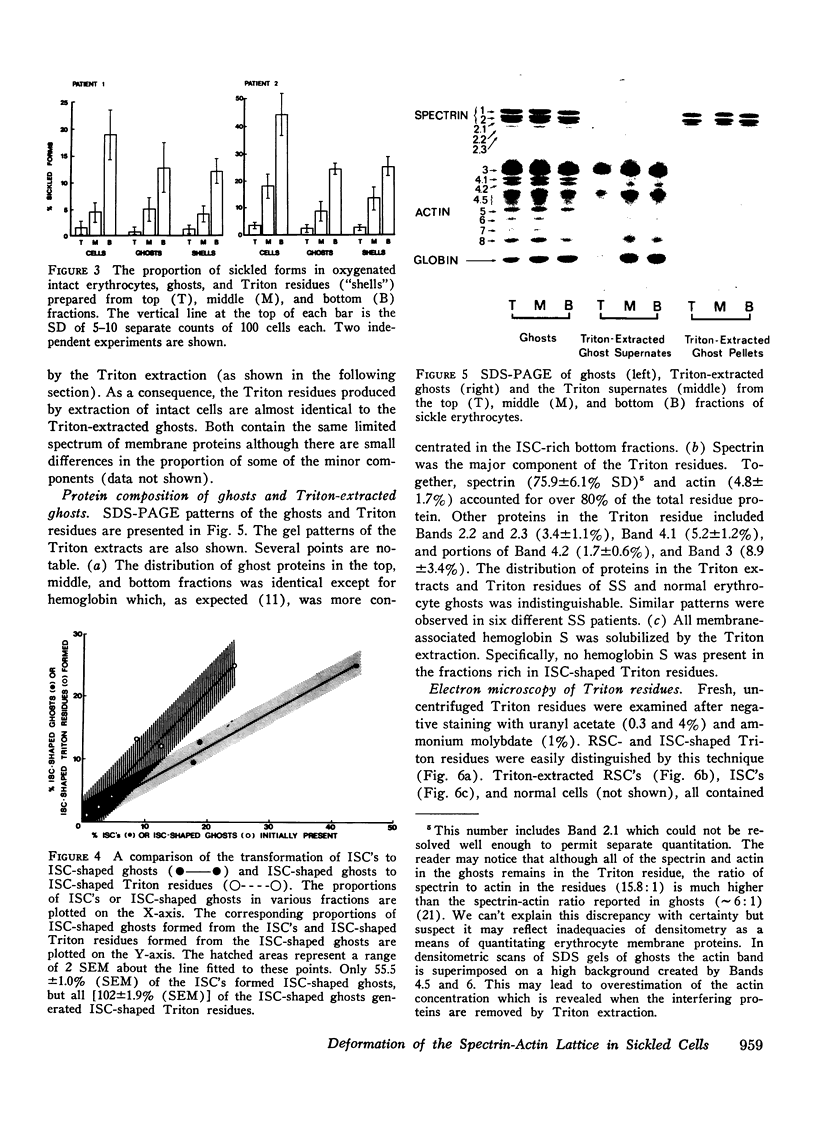
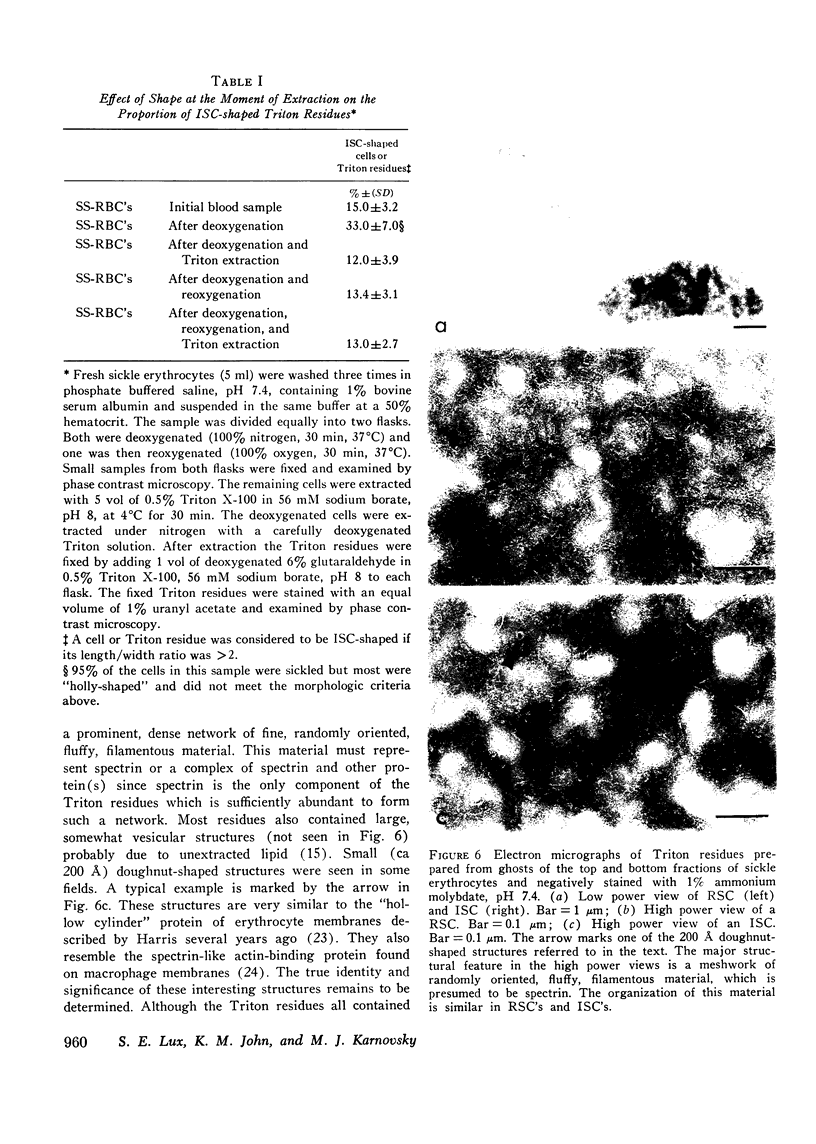
Irreversibly sickled cells (ISC's) are circulating erythrocytes in patients with sickle cell disease that retain a sickled shape even when oxygenated. Evidence points to a membrane defect that prevents the return of these cells to the normal biconcave shape. The erythrocyte membrane protein spectrin is believed to help control erythrocyte shape and deformability. Recent studies suggest that normally spectrin and an erythrocyte actin form a self-supporting, fibrillar, lattice-like network on the cytoplasmic membrane surface. When normal erythrocyte ghosts are extracted with Triton X-100 all the integral membrane proteins and most of the membrane lipids are removed, leaving a ghost-shaped residue composed principally of spectrin and actin. We concentrated ISC's from patients with sickle cell anemia and compared the morphology and protein composition of ghosts and Triton-extracted ghost residues prepared from these ISC's with similar preparations of reversibly sickable cells and normal cells. (a) Many ISC's formed ISC-shaped ghosts. (b) All ISC-shaped ghosts formed ISC-shaped Triton residues. (c) Spectrin, erythrocyte actin (Band 5), an unidentified Band 3 component, and Band 4.1 were the major protein components of the Triton residues. All membrane-associated sickle hemoglobin was removed by the Triton treatment. (d) No ISC-shaped ghosts or ISC-shaped Triton residues were formed when deoxygenated, sickled RSC's were lysed or Triton-extracted. ISC-shaped ghosts and Triton residues were never formed from normal cells. These observations suggest that a defect of the "spectrin-actin lattice" may be the primary abnormality of the ISC membrane. Since ISC's are rigid cells, the data support the postulate that spectrin is a major determinant of membrane deformability. Finally, they provide direct evidence that spectrin is important in determining erythrocyte shape.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Fairbanks G. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. I. A monovalent cation-stimulated reaction. Biochemistry. 1974 Dec 31;13(27):5507–5514. doi: 10.1021/bi00724a009. [DOI] [PubMed] [Google Scholar]
- Bertles J. F., Döbler J. Reversible and irreversible sickling: a distinction by electron microscopy. Blood. 1969 Jun;33(6):884–898. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. Isolation and characterization of a water-soluble protein from bovine erythrocyte membranes. Biochem Biophys Res Commun. 1971 Nov;45(4):1063–1070. doi: 10.1016/0006-291x(71)90445-1. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. I. The effects of protein removal. J Cell Biol. 1974 Dec;63(3):1018–1036. doi: 10.1083/jcb.63.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. II. Cyclic adenosine monophosphate-stimulated reactions. Biochemistry. 1974 Dec 31;13(27):5514–5521. doi: 10.1021/bi00724a010. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Beaven G. H. Properties of the high-molecular-weight protein (spectrin) from human-erythrocyte membranes. Eur J Biochem. 1975 Oct 15;58(2):403–409. doi: 10.1111/j.1432-1033.1975.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Harris J. R. Further studies on the proteins released from haemoglobin-free erythrocyte ghosts at low ionic strength. Biochim Biophys Acta. 1971 Mar 23;229(3):761–770. doi: 10.1016/0005-2795(71)90294-7. [DOI] [PubMed] [Google Scholar]
- Jensen M., Shohet S. B., Nathan D. G. The role of red cell energy metabolism in the generation of irreversibly sickled cells in vitro. Blood. 1973 Dec;42(6):835–842. [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Actin polymerisation induced by spectrin. Nature. 1975 Dec 25;258(5537):765–766. doi: 10.1038/258765a0. [DOI] [PubMed] [Google Scholar]
- Seakins M., Gibbs W. N., Milner P. F., Bertles J. F. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973 Feb;52(2):422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol. 1976 Mar;68(3):602–619. doi: 10.1083/jcb.68.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]