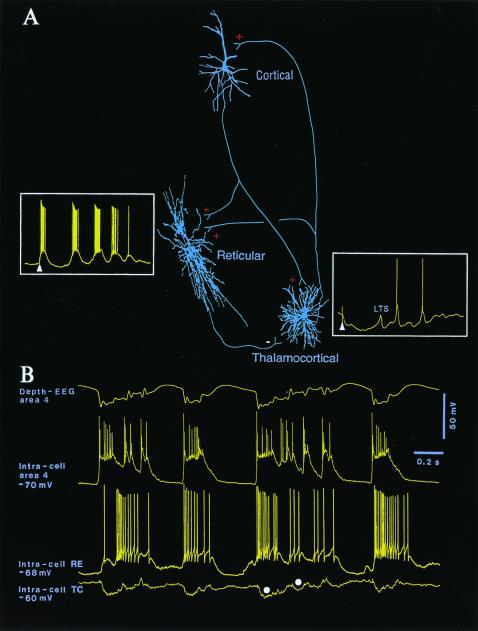
The projections of neocortical neurons to the thalamus are much denser than those from ascending pathways arising in the spinal cord and brainstem. Corticothalamic projections are estimated to outnumber thalamocortical ones by an order of magnitude. Thus, although the thalamus is the gateway of most sensory signals in their route to the cerebral cortex, the feedback corticothalamic projections are by far more massive. Until recently, however, the functional role of corticothalamic pathways remained unknown. The main reason was that, in the past, a series of studies using reversible cooling and ablation of cortical areas or, conversely, stimulation of those areas reported a variety of effects exerted by cortex on the thalamus, from depressed to enhanced activity, or simply lack of any definite result. The first advances in this domain stemmed from the recognition of three major neuronal types in the thalamus, with the consequence that the excitatory or inhibitory sign of cortical actions actually depends on a delicate balance between a prevalent effect exerted on one or the other of these thalamic neuronal classes. Two of these three neuronal types in the thalamus are cortically projecting [relay or thalamocortical (TC)] neurons that are glutamatergic (thus excitatory), and reticular (RE) neurons that are GABAergic (inhibitory) and do not project to cortex but back to TC neurons, closing a recurrent inhibitory loop. The most important input sources of RE neurons are neocortical and TC neurons (Fig. 1A). The third cellular type, local-circuit GABAergic neurons, is found in all dorsal thalamic nuclei of felines and primates (as well as in the visual thalamus of rodents) in sizeable (≈25%) proportions (1). Although local inhibitory interneurons play important roles in thalamic function, they are not usually incorporated in conventional diagrams because, for practical factors, most in vitro studies are conducted in other dorsal thalamic nuclei of rodents.
Figure 1.
Functional relations between corticothalamic, thalamic reticular (RE), and thalamocortical (TC) neurons. (A) Three neurons (cortical, RE, and TC) were intracellularly recorded and stained in cats. Signs of excitation and inhibition are indicated by plus and minus. For the sake of simplicity, local-circuit inhibitory neurons in cortex and thalamus are not illustrated. (Insets) The response of RE and TC neurons to cortical stimulation (arrowheads point to stimulus artifacts). The GABAergic RE neuron responded to cortical stimulation with a high-frequency spike-burst, followed by a sequence of spindle waves on a depolarizing envelope (membrane potential, −68 mV). The TC neuron responded to cortical stimulation (arrowhead) with a biphasic IPSP, leading to a low-threshold spike (LTS) and a sequence of hyperpolarizing spindle waves (membrane potential, −70 mV). (B) Relations between cortical (area 4), RE, and TC neurons of cat during spontaneously occurring, cortically generated seizure with polyspike-wave (PSW) complexes at 2 Hz. Note IPSPs in TC neuron (filled circles) in close time relation with spike-bursts fired by RE neuron, driven from cortex. Recordings are modified from refs. 26 and 27.
The issue addressed by Golshani et al. (2) in this issue of PNAS is that, although all corticothalamic axons are glutamatergic (thus exerting excitatory actions on both RE and TC neurons), the effect of a natural or artificial corticofugal volley is opposite on each of these two neuronal types. Synchronous cortical volleys (which occur naturally during slow-wave sleep when neurons exhibit highly coherent activity) or electrical stimuli produce excitation and rhythmic spike-bursts over a depolarizing envelope in RE neurons, whereas TC neurons simultaneously display rhythmic and prolonged inhibitory postsynaptic potentials (IPSPs), occasionally followed by rebound excitations (Fig. 1A). After excitotoxic lesions of RE neurons or transections separating them from the remaining thalamus, the prolonged IPSP-rebound sequences, which underlie sleep spindle oscillations in TC neurons, disappear; instead, TC neurons receive numerous, short-lasting IPSPs from local interneurons (3). This result and others qualify the RE nucleus as the pacemaker of spindles. Pure signs of cortically elicited excitation in TC neurons may be seen only after removing the RE nucleus from this complex circuitry. These data suggest that the cortical projection to RE neurons is more powerful than the action it exerts on TC neurons. In other words, the bisynaptic inhibition of TC neurons, induced by cortex through a prior synaptic relay on GABAergic RE neurons, overcomes the direct excitation of TC neurons. The recent results of Golshani et al. (2) provide evidence that the numbers of glutamate receptor subunits GluR4 are 3.7 higher at corticothalamic synapses in RE neurons, compared with TC neurons, and that the mean peak amplitude of corticothalamic excitatory postsynaptic currents (EPSCs) is about 2.5 higher in RE, than in TC, neurons. These findings are extremely important for both neurophysiologists and computational neurobiologists, who have long waited for a quantitative measure of the power exerted by the excitatory synaptic input from cortex to RE and TC neurons.
Why are the present data of Golshani et al. (2) important? Because a series of natural phenomena, occurring during alertness but especially in the sleeping brain, as well as during abnormally synchronized events that characterize paroxysmal states, depend on varying functional states of RE neurons, which have different consequences on TC neurons. The most potent effects are exerted by RE neurons when they fire prolonged, rhythmic, high-frequency spike-bursts, which are the signature of these GABAergic neurons during slow-wave sleep, whereas the same neurons discharge tonically, in the single-spike mode in the waking and the state of sleep with rapid eye movements (4). The tonic activity of RE neurons during waking may assist in suppressing background activity of TC neurons and in enhancing the transfer of incoming signals (5). By contrast, during slow-wave sleep, and even more so during some types of electrical seizures that develop from sleep patterns, the spike-bursts fired by RE neurons induce greater postsynaptic inhibitory responses in TC neurons than those elicited by single spikes. The synchronous discharges of corticothalamic neurons during the slow sleep oscillation (6) impinge on, and excite, RE neurons, eventually inducing prolonged hyperpolarizations and rebound spike-bursts in target TC neurons (7).
This sequence of events, from cortex to thalamus, first to RE neurons and then to TC neurons, underlies the coalescence of different sleep oscillatory types. Instead of simple rhythms, arising from simple circuits, as investigated in isolated thalamic or cortical slices maintained in vitro, the intact brains of animals and humans display complex wave-sequences, comprising different types of low-frequency and high-frequency oscillations, which are produced by operations in interacting corticothalamic neuronal loops, under the control of generalized modulatory systems. As acknowledged by in vitro investigators, small pieces of nervous tissue are not adequate to support oscillations for prolonged periods of time (8). And some neuronal properties described in slices may be different from those seen in the living organism (9).
As an illustration of the role played by corticothalamic synaptic connections and their stronger impact on RE neurons than on TC neurons (2), I will refer to several phenomena that occur during states of vigilance and during seizures. The first aspect concerns spindle oscillations (7 to 14 Hz), a hallmark of early sleep stages. Unlike their systematic propagation in thalamic slices (10), spindle sequences are nearly simultaneous over widespread territories of the thalamus and cortex of animals and humans (11). It was demonstrated that these contrasting results between in vitro and in vivo experiments were due to the absence of corticothalamic projections in a thalamic slice as, after ablation of neocortex, the quasi-simultaneity of spindles is lost in the ipsilateral thalamus (11). On the contrary, increasing the excitability of cortical neurons leads to enhanced synchrony of thalamic spindles. Experimental data combined with computational models demonstrate that this increased activity of corticothalamic neurons operates through inhibition of TC neurons via RE neurons, with the prediction that corticothalamic feedback should lead to large-scale coherent activity by recruiting thalamic circuitry through prevalent projections to RE nucleus (12). The network structure and conductance values used in those computational models would have greatly benefited from the knowledge acquired from the present data by Golshani et al. (2), conducted in slices with intact corticothalamic innervation, that quantify the difference in efficacy of corticothalamic EPSCs in RE and TC neurons.
The new results (2) also shed light on controversial ideas about the initiation site (cortex or thalamus) and the thalamic processes implicated in the genesis of paroxysmal events consisting of spike-wave (SW) or polyspike-wave (PSW) complexes at about 2–3 Hz (hereafter called SW seizures). This electrographic pattern seen in experimental studies is similar to that observed in clinical absence (petit-mal) epileptic seizures. In the encephalopathy known as Lennox-Gastaut syndrome (13), SW seizures are often combined with fast runs at 10–15 Hz (14). SW seizures were once thought to originate in the thalamus and, in view of the generalized seizures that appear suddenly on the visual inspection of electroencephalogram (EEG), they were ascribed to the so-called “centrencephalic system” (15). It is difficult to envisage such a deeply located pacemaker of SW seizures, because there are no bilaterally projecting thalamic neurons. Moreover, the sudden appearance of generalized SW seizures is disputed in view of topographical analyses of SW complexes in humans, showing that the “spike” component propagates with very short time lags (that cannot be estimated visually) from one hemisphere to another (16). In fact, experiments using multisite, extra- and intracellular recordings show that neocortical neurons become progressively entrained into the seizures, indicating that the buildup of SW seizures obeys the rule of short-scale and long-scale synaptic circuits (17). After a period when cyclic neuronal processes in intrathalamic circuits investigated in slices were thought to be responsible for absence SW seizures, there is now evidence from intact-brain preparations that typical SW seizures are initiated in neocortex and that they spread to the thalamus only after a few seconds (18).
What is the behavior of TC neurons during cortically generated SW seizures? Here, the new data by Golshani et al. (2) are again of crucial importance. Indeed, it was demonstrated in vivo, both in cats (19) and subsequently in a genetic model of absence epilepsy in rats (20), that, during cortically generated SW seizures, a majority of TC neurons are steadily hyperpolarized and display phasic IPSPs. These inhibitory potentials are mediated by RE neurons that faithfully follow each paroxysmal depolarizing shift of cortical neurons (ref. 19; Fig. 1B). This finding fully corroborates the data in the present paper (2) showing that cortically elicited weak excitation of TC neurons is overwhelmed by strong inhibition of the same neurons, mediated by cortico-RE projections. The role of corticothalamic projections in the generation of SW seizures (19) is now confirmed in a recent series of in vitro studies showing that bursting at 3 Hz in thalamic neurons can no longer be evoked in the thalamus after removal of cortex (21, 22) and that corticofugal volleys induce thalamic activity at 3–4 Hz (23, 24). These results stand in contrast to the previous hypothesis that thalamic networks are primarily (or even alone) implicated in the genesis of SW seizures.
Of course, besides these prevalent inhibitory projections from cortex to TC neurons, which are mediated through RE neurons during highly synchronized activities, excitatory cortical actions on TC neurons can also be revealed when RE neurons fire in the single-spike mode (4), and their impact on TC neurons is less pronounced than when they fire long spike-bursts. This scenario was much less intensively investigated.
What is gratifying about the present study (2) and a parallel study of the team led by Jones (25) is that they provide quantitative data, at both electron microscopic and electrophysiological levels, on the synaptic weight of the major projections reaching GABAergic RE neurons, a key structure implicated in normal and paroxysmal oscillatory activities.
Acknowledgments
Personal experiments mentioned in this commentary were supported by grants from the Medical Research Council of Canada and Human Frontier Science Program.
Footnotes
See companion article on page 4172.
References
- 1.Jones E G. The Thalamus. New York: Plenum; 1985. p. 935. [Google Scholar]
- 2.Golshani P, Liu X-B, Jones E G. Proc Natl Acad Sci USA. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. . (First Published February 27, 2001; 10.1073/pnas.061013698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steriade M, Deschênes M, Domich L, Mulle C. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M, Domich L, Oakson G. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren R A, Jones E G. Exp Brain Res. 1994;100:215–226. doi: 10.1007/BF00227192. [DOI] [PubMed] [Google Scholar]
- 6.Steriade M, Nuñez A, Amzica F. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steriade M, Contreras D, Curró Dossi R, Nuñez A. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chagnac-Amitai Y, Connors B W. J Neurophysiol. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- 9.Steriade M. The Intact and Sliced Brain. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 10.Kim U, Bal T, McCormick D A. J Neurophysiol. 1995;74:1301–1323. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- 11.Contreras D, Destexhe A, Sejnowski T J, Steriade M. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 12.Destexhe A, Contreras D, Steriade M. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- 13.Niedermeyer E. In: Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Niedermeyer E, Lopes da Silva F, editors. Baltimore: Williams & Wilkins; 1999. pp. 235–260. [Google Scholar]
- 14.Steriade M, Amzica F, Neckelmann D, Timofeev I. J Neurophysiol. 1998;80:1465–1479. doi: 10.1152/jn.1998.80.3.1456. [DOI] [PubMed] [Google Scholar]
- 15.Penfield W, Jasper H H. Epilepsy and the Functional Anatomy of the Human Brain. Brown, Boston: Little; 1954. [Google Scholar]
- 16.Lemieux J F, Blume W T. Brain Res. 1986;373:275–287. doi: 10.1016/0006-8993(86)90342-2. [DOI] [PubMed] [Google Scholar]
- 17.Steriade M, Amzica F. J Neurophysiol. 1994;72:2051–2069. doi: 10.1152/jn.1994.72.5.2051. [DOI] [PubMed] [Google Scholar]
- 18.Neckelmann D, Amzica F, Steriade M. J Neurophysiol. 1998;80:1480–1494. doi: 10.1152/jn.1998.80.3.1480. [DOI] [PubMed] [Google Scholar]
- 19.Steriade M, Contreras D. J Neurosci. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinault D, Leresche N, Charpier S, Deniau J M, Marescaux C, Vergnes M, Crunelli V. J Physiol (London) 1998;509:449–456. doi: 10.1111/j.1469-7793.1998.449bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao C Q, Coulter D A. J Neurophysiol. 1997;77:2661–2676. doi: 10.1152/jn.1997.77.5.2661. [DOI] [PubMed] [Google Scholar]
- 22.Golshani P, Jones E G. J Neurosci. 1999;19:2865–2875. doi: 10.1523/JNEUROSCI.19-08-02865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bal T, Debay D, Destexhe A. J Neurosci. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumenfeld H, McCormick D A. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, X.-B., Bolea, S., Golshani, P. & Jones, E. G. (2001) Thalamus1, in press.
- 26.Contreras D, Steriade M. J Physiol (London) 1996;490:159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lytton W W, Contreras D, Destexhe A, Steriade M. J Neurophysiol. 1997;77:1679–1696. doi: 10.1152/jn.1997.77.4.1679. [DOI] [PubMed] [Google Scholar]