Abstract
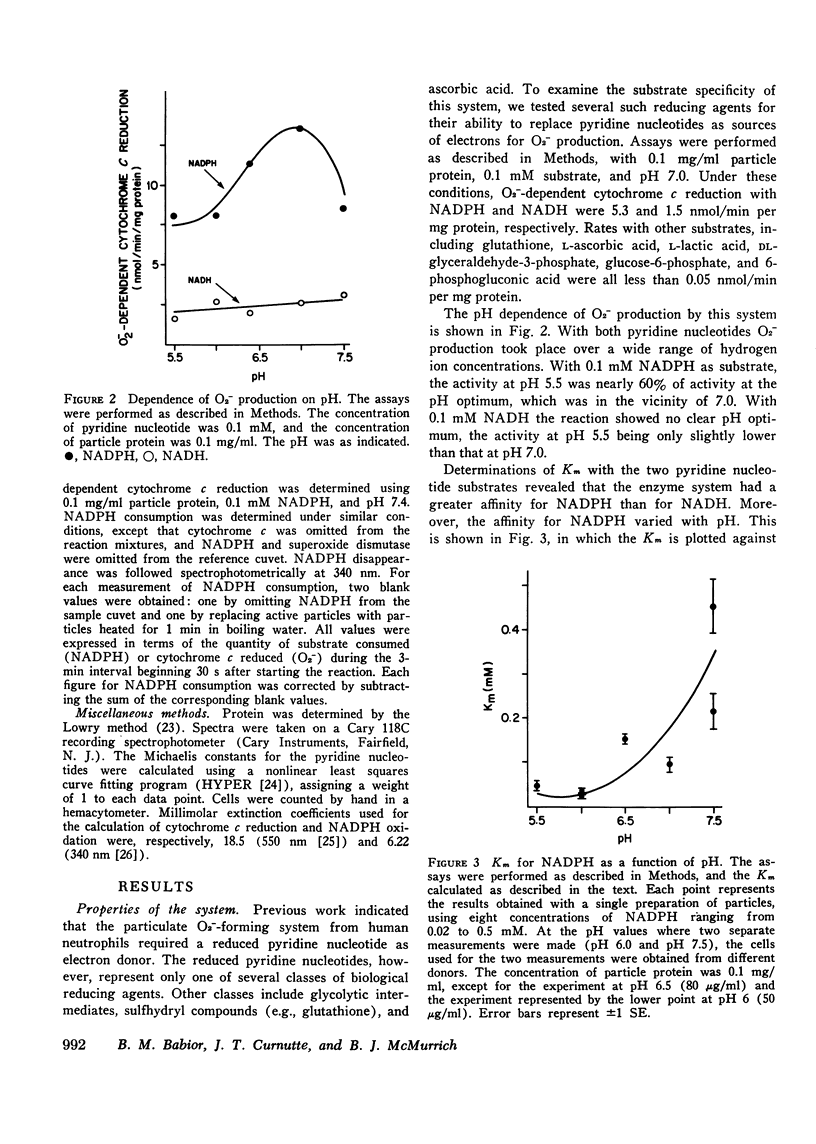
Studies were performed to characterize the previously reported particulate O2--forming system from human neutrophils. Of eight reducing agents examined, including glutathione, ascorbic acid, and intermediates of the glycolytic and hexose monophosphate shunt pathways, only the pyridine nucleotides could serve as electron donors. At 0.1 mM pyridine nucleotide, O2- production was relatively independent of pH. The Km for NADH was approximately 0.7 mM regardless of pH, while with NADPH the Km varied from 0.02 mM at pH 6.0 to 0.3 mM at pH 7.5. The molar ratio of NADPH oxidized to O2- produced was consistent with the reaction: NADPH + 2 O2- leads to NADP+ H+; the product nucleotide was shown enzymatically to be NADP. O2- production was not inhibited by CN-, Na-, EDTA, or 1,10-phenanthroline. Particulate O2- production accounted for 35% of the oxygen taken up during the respiratory burst by an equivalent number of intact neutrophils. Greatly diminished O2- production was seen with particles prepared from cells obtained from three patients with chronic granulomatous disease, with 2.5 mM NADPH as electron donor. With 5.0 mM NADH similar observations were made with particles from two of the patients, but with this nucelotide, O2- production was only slightly reduced in the third case. The evidence available suggests that this particulate O2- -forming system is the one responsible for the respiratory burst in activated neutrophils. The relationship between this system and other O2- -forming system found in human neutrophils is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes B. S. Pyridine nucleotide-dependent superoxide production by a cell-free system from human granulocytes. J Clin Invest. 1975 Oct;56(4):1035–1042. doi: 10.1172/JCI108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Curnutte J. T., Karnovsky M. L., Babior B. M. Manganese-dependent NADPH oxidation by granulocyte particles. The role of superoxide and the nonphysiological nature of the manganese requirement. J Clin Invest. 1976 Apr;57(4):1059–1067. doi: 10.1172/JCI108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. Chronic granulomatous disease--pieces of a cellular and molecular puzzle. Fed Proc. 1973 Apr;32(4):1527–1533. [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugh T. H., 2nd Singlet oxygen: a unique microbicidal agent in cells. Science. 1973 Oct 5;182(4107):44–45. doi: 10.1126/science.182.4107.44. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]




