Abstract
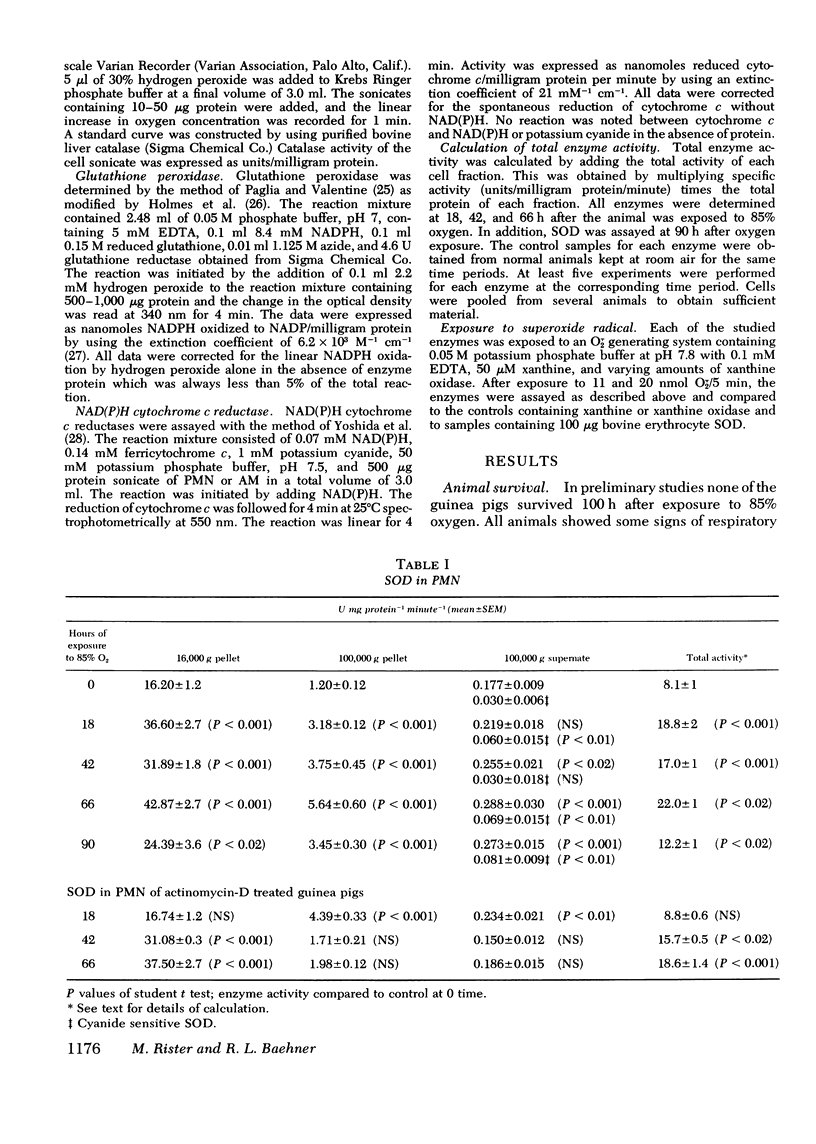
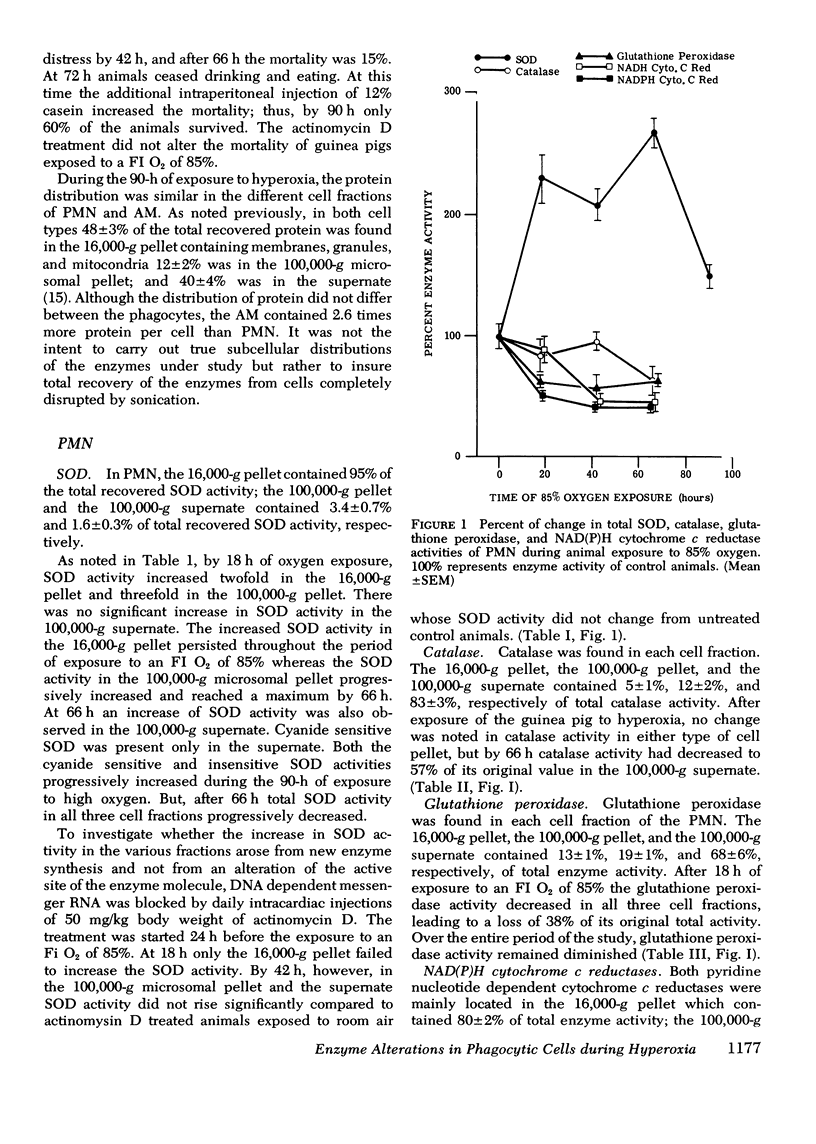
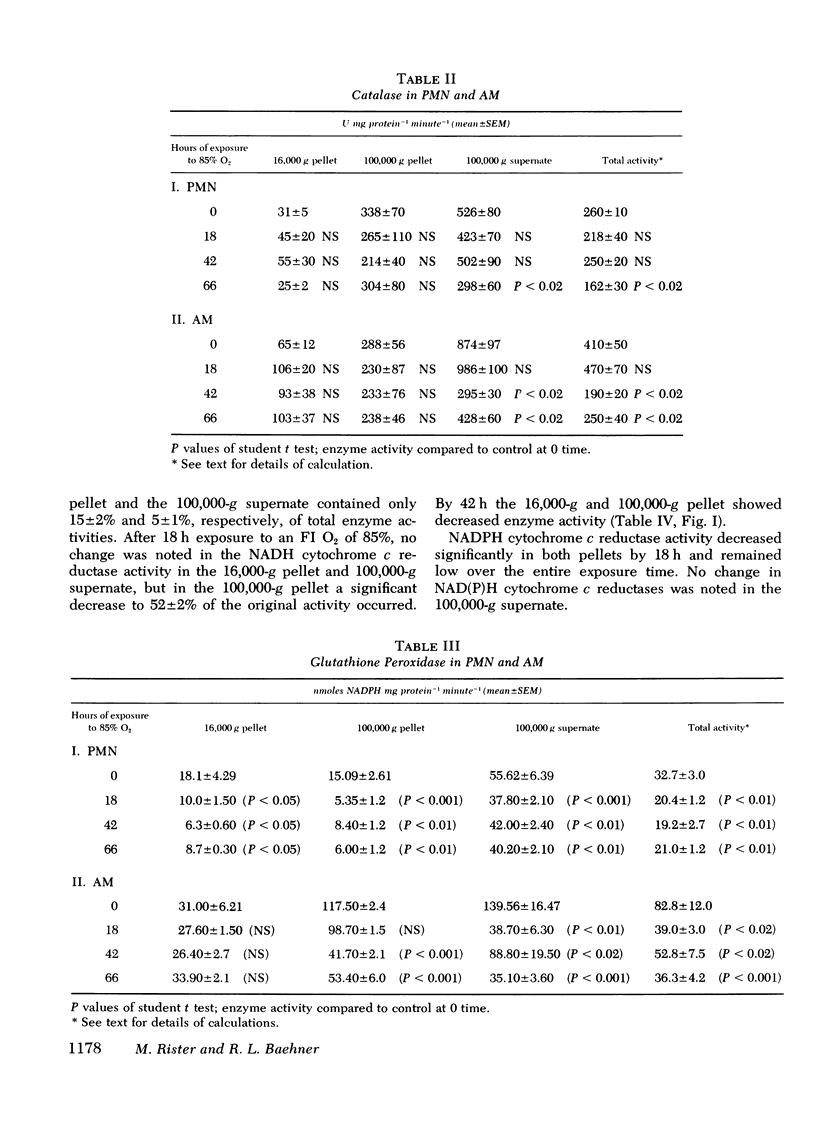
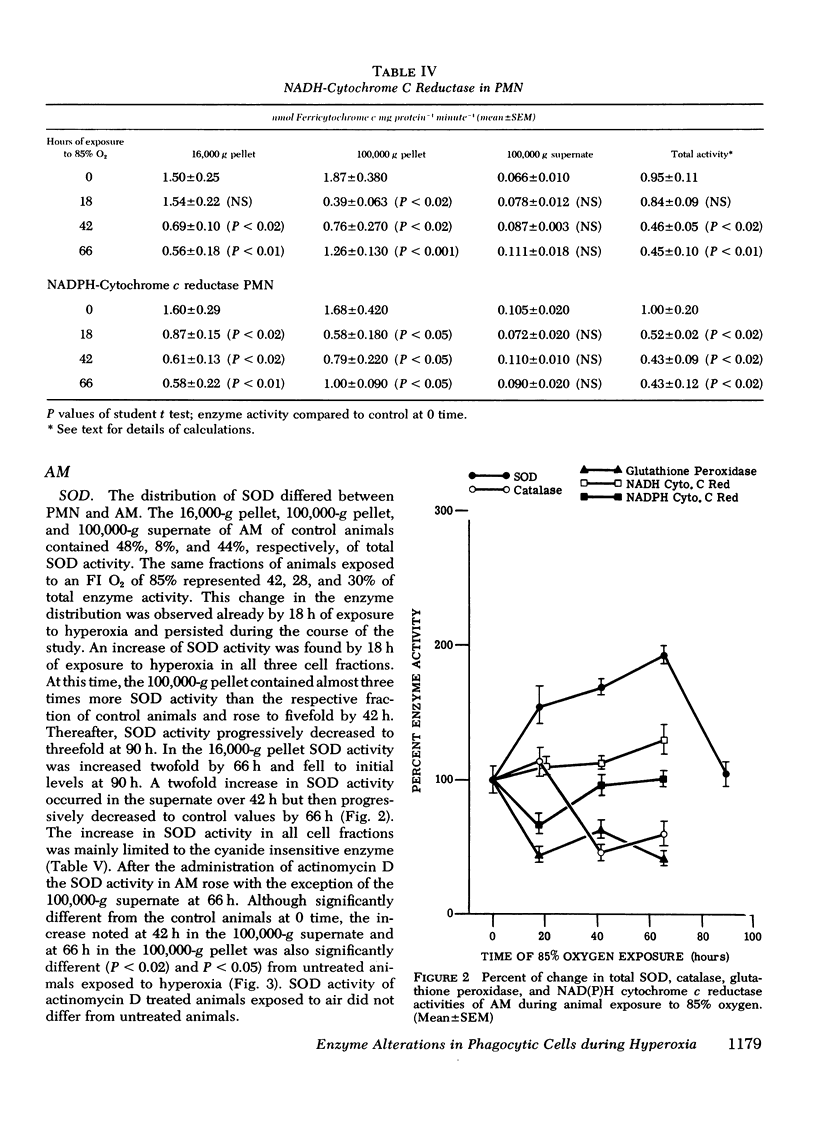
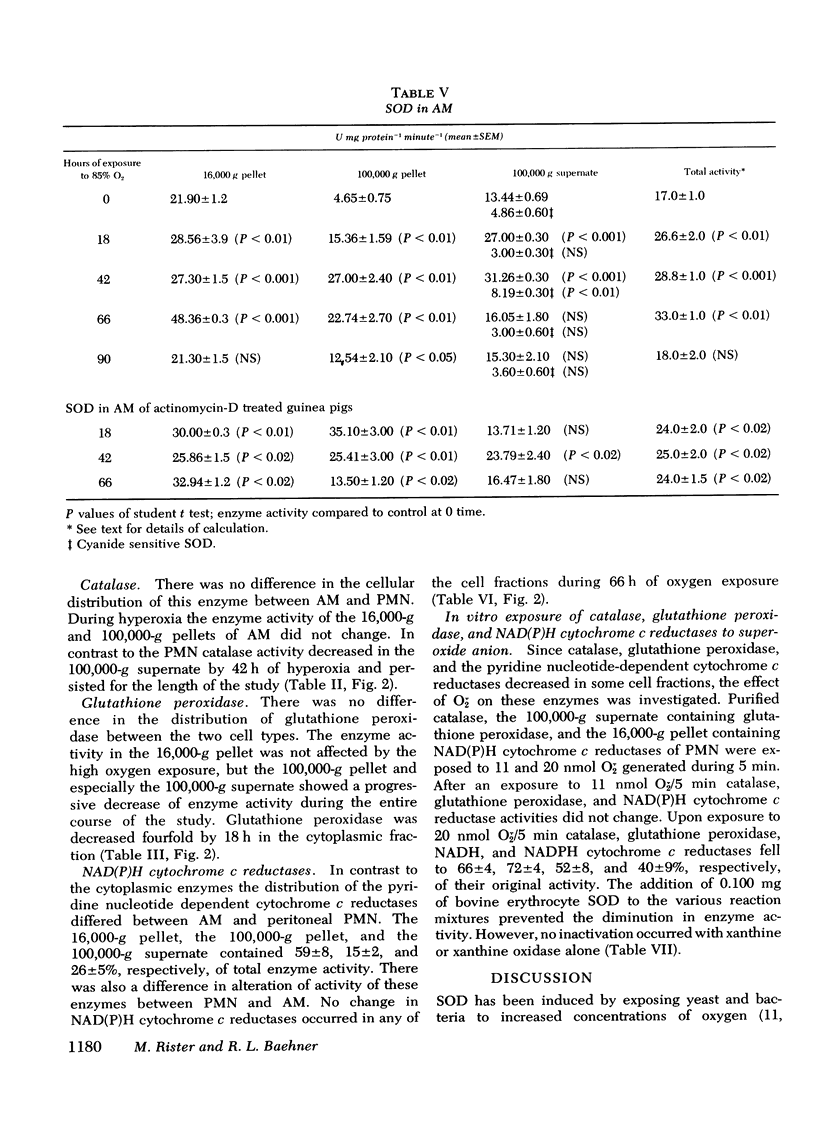
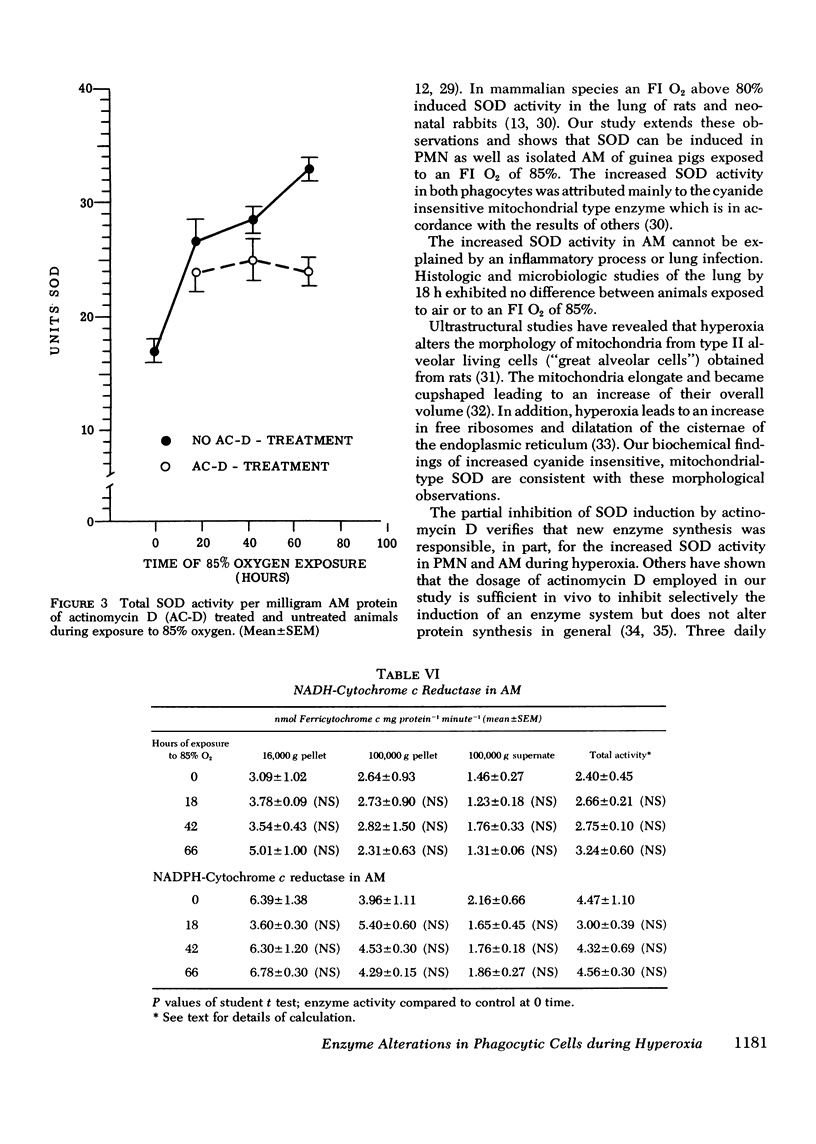
Superoxide dismutase, catalase, glutathione peroxidase and NAD(P)H cytochrome c reductase were quantitated in polymorphonuclear leukocytes (PMN) and alveolar macrophages (AM) obtained from guinea pigs exposed up to 90 h to 85% oxygen. PMN and AM were sonicated and separated into a 16,000-g pellet, a 100,000-g pellet, and a 100,00-g supernate. Superoxide dismutase activity increased in both cells within 18 h, persisted for 66 h and decreased by 90 h. The highest rate of increase was in the 100,000-g pellet containing 3.4% of total enzyme activity in PMN but 28% in AM. The enzyme induction in PMN and AM was partially inhibited by daily intracardiac injections of 50 mg/kg actinomycin D. During oxygen exposure, catalase activity in PMN and AM decreased to 60% of its original activity, and gluthathione peroxidase was reduced in PMN to 60% and in AM to 20% of control values. Although NAD(P)H cytochrome c reductase decreased to 50% in PMN, no change was noted in AM. Upon exposure to superoxide anion, purified catalase, the glutathione peroxidase of the 100,000-g supernate, NADH, and NADPH cytochrome c reductases of the 16,000-g pellet decreased to 66+/-5%, 72+/-4%, 52+/-8%, and 40+/-9%, respectively, of their original activity. This inactivation was prevented by 0.1 mg superoxide dismutase. These in vitro observations could explain the decreased catalase and glutathione peroxidase activity demonstrated in vivo that may lead to an intracellular accumulation of hydrogen peroxide. Increased hydrogen peroxide concentrations have been found to inactivate superoxide dismutase thus impairing the first defense mechanism against superoxide anion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autor A. P., Frank L., Roberts R. J. Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res. 1976 Mar;10(3):154–158. doi: 10.1203/00006450-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- Brain J. D., Frank R. Alveolar macrophage adhesion: wash electrolyte composition and free cell yield. J Appl Physiol. 1973 Jan;34(1):75–80. doi: 10.1152/jappl.1973.34.1.75. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Cockle S. A., Fielden E. M., Roberts P. B., Rotilio G., Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J. 1974 Apr;139(1):43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Clark J. M. The toxicity of oxygen. Am Rev Respir Dis. 1974 Dec;110(6 Pt 2):40–50. doi: 10.1164/arrd.1974.110.6P2.40. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. The autoxidation of red cells. Br J Haematol. 1971 May;20(5):457–461. doi: 10.1111/j.1365-2141.1971.tb07060.x. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Bergamini R., Briggs R. G. Observations on the mechanism of the oxygen/dialuric acid-induced hemolysis of vitamin e-deficient rat red blood cells and the protective roles of catalase and superoxide dismutase. Arch Biochem Biophys. 1975 Jul;169(1):160–167. doi: 10.1016/0003-9861(75)90329-x. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Gaber B. P. Anion binding to bovine erythrocyte superoxide dismutase. Evidence for multiple binding sites with qualitatively different properties. J Biol Chem. 1972 Jan 10;247(1):60–65. [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973 Sep;158(1):396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Oxygen: boon and bane. Am Sci. 1975 Jan-Feb;63(1):54–59. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gee J. B., Kaskin J., Duncombe M. P., Vassallo C. L. The effects of ethanol on some metabolic features of phagocytosis in the alveolar macrophage. J Reticuloendothel Soc. 1974 Jan;15(1):61–68. [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel C. E. The effects of hyperoxia on red cells as related to tocopherol deficiency. Ann N Y Acad Sci. 1972 Dec 18;203:163–171. doi: 10.1111/j.1749-6632.1972.tb27870.x. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Chance B. The properties of hydrogen peroxide production under hyperoxic and hypoxic conditions of perfused rat liver. Biochem J. 1975 Jan;146(1):53–65. doi: 10.1042/bj1460053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Sugano T., Chance B. Optical measurement of the catalase-hydrogen peroxide intermediate (Compound I) in the liver of anaesthetized rats and its implication to hydrogen peroxide production in situ. Biochem J. 1975 Jan;146(1):67–77. doi: 10.1042/bj1460067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Pratt P. C. Pathology of pulmonary oxygen toxicity. Am Rev Respir Dis. 1974 Dec;110(6 Pt 2):51–57. doi: 10.1164/arrd.1974.110.6P2.51. [DOI] [PubMed] [Google Scholar]
- Resnick J. S., Brown D. M., Vernier R. L. Oxygen toxicity in fetal organ culture. II. The developing lung. Lab Invest. 1974 Dec;31(6):665–677. [PubMed] [Google Scholar]
- Rister M., Baehner R. L. A comparative study of superoxide dismutase activity in polymorphonuclear leukocytes, monocytes, and alveolar macrophages of the guinea pig. J Cell Physiol. 1976 Mar;87(3):345–355. doi: 10.1002/jcp.1040870310. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R. M., Wittner M., Lenger M. Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab Invest. 1969 Jun;20(6):516–528. [PubMed] [Google Scholar]
- Rozenszajn L., Leibovich M., Shoham D., Epstein J. The esterase activity in megaloblasts, leukaemic and normal haemopoietic cells. Br J Haematol. 1968 Jun;14(6):605–610. doi: 10.1111/j.1365-2141.1968.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman H. A., Fridovich I. Editorial: Oxygen toxicity. Introduction to a protective enzyme: superoxide dismutase. Circulation. 1973 Nov;48(5):921–923. doi: 10.1161/01.cir.48.5.921. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Oxygen effect on lung cells. Arch Intern Med. 1971 Jul;128(1):54–56. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Yamamoto E., Wittner M., Rosenbaum R. M. Resistance and susceptibility to oxygen toxicity by cell types of the gas-blood barrier of the rat lung. Am J Pathol. 1970 Jun;59(3):409–436. [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kumaoka H., Sato R. Studies on the microsomal electron-transport system of anaerobically grown yeast. I. Intracellular localization and characterization. J Biochem. 1974 Jun;75(6):1201–1210. doi: 10.1093/oxfordjournals.jbchem.a130503. [DOI] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]


