Abstract
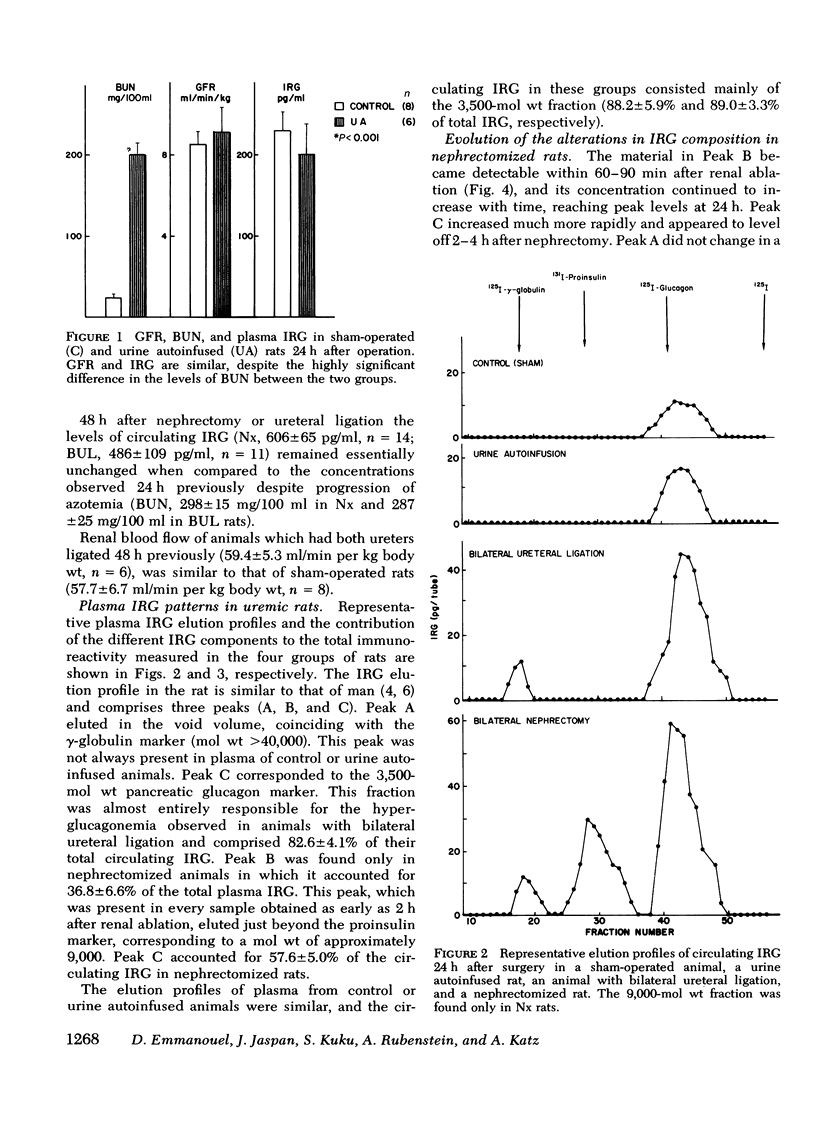
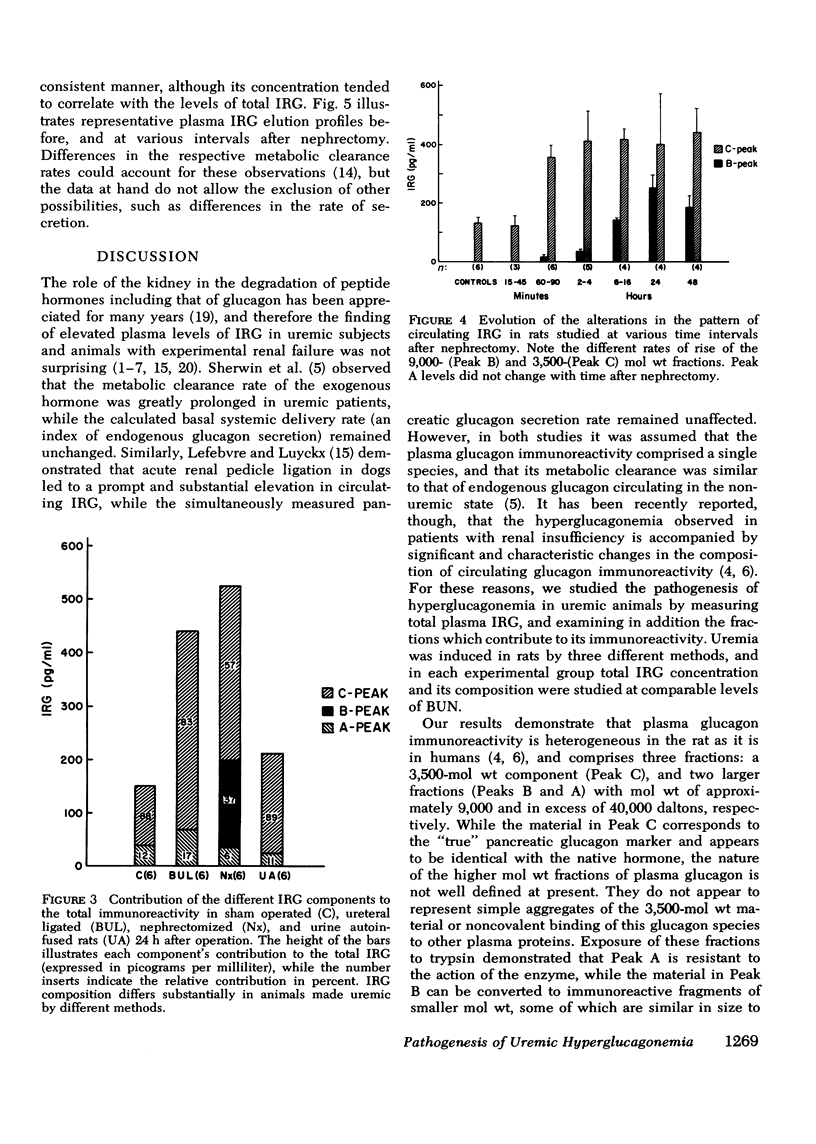
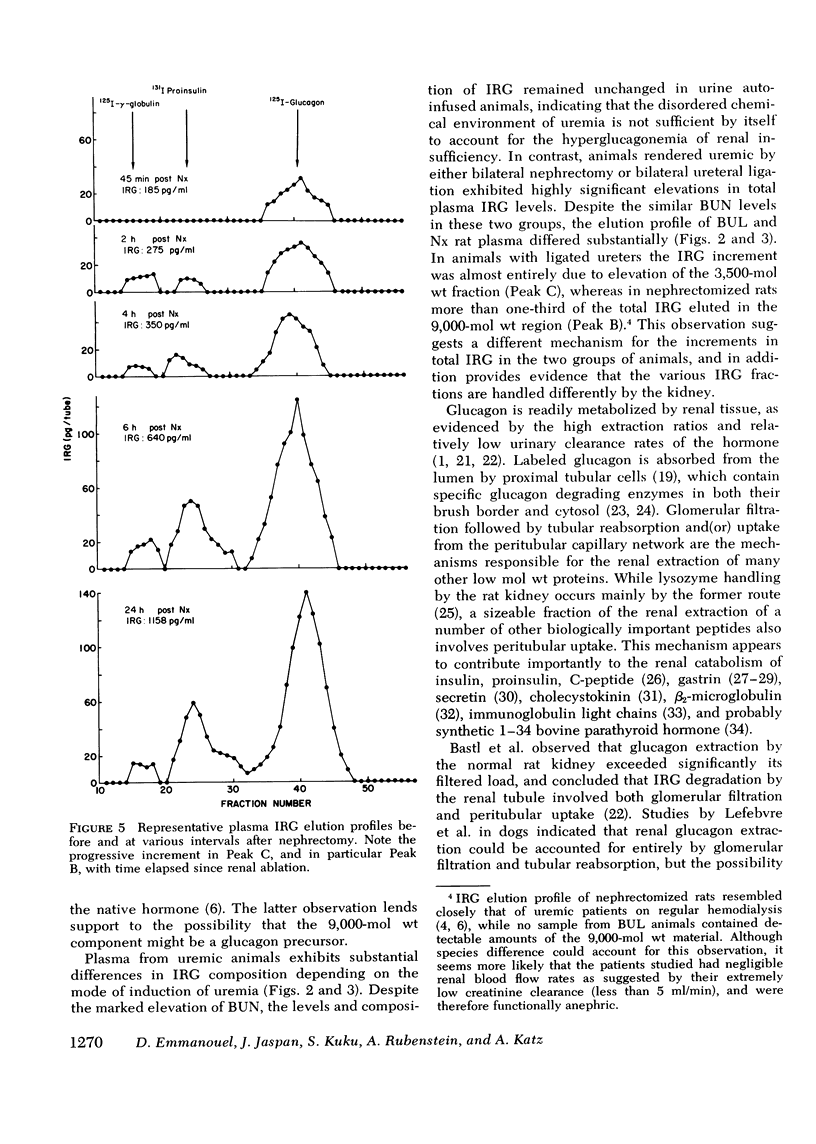
The pathogenesis of hyperglucagonemia and of the alterations in the pattern of circulating immunoreactive glucagon (IRG) associated with renal insufficiency was studied in rats in which a comparable degree of uremia was induced by three different methods, i.e., bilateral nephrectomy, bilateral ureteral ligation, and urine autoinfusion. Nephrectomized and ureteral-ligated rats were markedly hyperglucagonemic (575 +/- 95 pg/ml and 492 +/- 54 pg/ml, respectively), while IRG levels of urine autoinfused animals (208 +/- 35 pg/ml) were similar to those of control rats (180 +/- 26 pg/ml), indicating that uremia per se does not account for the hyperglucagonemia observed in renal failure. Similarly, plasma IRG composition in this group of animals was indistinguishable from that of controls, in which 88.2 +/- 5.9% of total IRG consisted of the 3,500-mol wt fraction. The same component was almost entirely responsible (82.6 +/- 4.1%) for the hyperglucagonemia observed in ligated rats, while it accounted for only 57.6 +/- 5.0% of the circulating IRG in nephrectomized animals. In the latter group, 36.8 +/- 6.6% of total IRG had a mol wt of approximately 9,000, consistent with a glucagon precursor. This peak was present in samples obtained as early as 2 h after renal ablation and its concentration continued to increase with time reaching maximal levels at 24 h. These results confirm that the kidney is a major site of glucagon metabolism and provide evidence that the renal handling of the various circulating IRG components may involve different mechanisms. Thus, the metabolism of the 3,500-mol wt fraction is dependent upon glomerular filtration, while the uptake of the 9,000-mol wt material can proceed in its absence, as long as renal tissue remains adequately perfused. This finding suggests that the 9,000-mol wt component may be handled by peritubular uptake.
Full text
PDF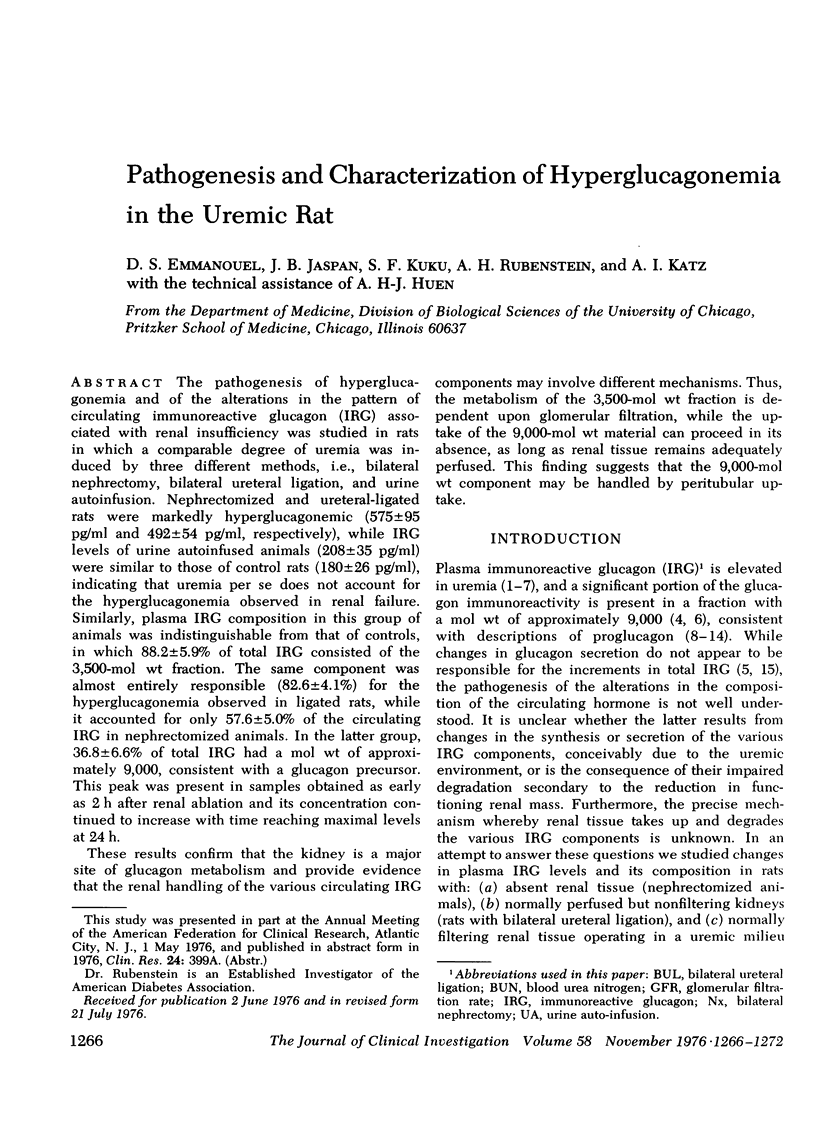



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier G. M., Conrad M. E. Catabolsm of human beta-2-microglobulin by the rat kidney. Am J Physiol. 1969 Nov;217(5):1359–1362. doi: 10.1152/ajplegacy.1969.217.5.1359. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Atkins C., Hull A. R., Knochel J. P. Hyperglucagonemia in uremia: reversal by renal transplantation. Ann Intern Med. 1975 Apr;82(4):525–528. doi: 10.7326/0003-4819-82-4-525. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. A., Reeder D. D., Hjelmquist U. B., Brandt E. N., Jr, Thompson J. C. Renal inactivation of endogenous gastrin in dogs. Arch Surg. 1973 Jun;106(6):851–854. doi: 10.1001/archsurg.1973.01350180085024. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Clendinnen B. G., Davidson W. D., Reeder D. D., Jackson B. M., Thompson J. C. Renal uptake and excretion of gastrin in the dog. Surg Gynecol Obstet. 1971 Jun;132(6):1039–1043. [PubMed] [Google Scholar]
- Daubresse J. C., Lerson G., Plamteux G., Rorive G., Luyckx A. S., Lefebvre P. J. Lipids and lipoproteins in chronic uraemia. A study of the influence of regular haemodialysis. Eur J Clin Invest. 1976 Mar 31;6(2):159–166. doi: 10.1111/j.1365-2362.1976.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Grace S. G., Davidson W. D., State D. Renal mechanisms for removal of gastrin from the circulation. Surg Forum. 1974;25(0):323–325. [PubMed] [Google Scholar]
- Hellerström C., Howell S. L., Edwards J. C., Andersson A., Ostenson C. G. Biosynthesis of glucagon in isolated pancreatic islets of guinea pigs. Biochem J. 1974 Apr;140(1):13–21. doi: 10.1042/bj1400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska K. A., Kopelman R., Rutherford W. E., Klahr S., Slatopolsky E., Greenwalt A., Bascom T., Markham J. Metabolism in immunoreactive parathyroid hormone in the dog. The role of the kidney and the effects of chronic renal disease. J Clin Invest. 1975 Jul;56(1):39–48. doi: 10.1172/JCI108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kurtz T. W., Preuss H. G., Weller J. M. Measurement of renal blood flow in the rat. Proc Soc Exp Biol Med. 1975 Jun;149(2):470–472. doi: 10.3181/00379727-149-38829. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuku S. F., Jaspan J. B., Emmanouel D. S., Zeidler A., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon. Circulating components in normal subjects and patients with chronic renal failure. J Clin Invest. 1976 Sep;58(3):742–750. doi: 10.1172/JCI108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuku S. F., Zeidler A., Emmanouel D. S., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon: patterns in patients with chronic renal failure and diabetes. J Clin Endocrinol Metab. 1976 Jan;42(1):173–176. doi: 10.1210/jcem-42-1-173. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S. Effect of acute kidney exclusion by ligation of renal arteries on peripheral plasma glucagon levels and pancreatic glucagon production in the anesthetized dog. Metabolism. 1975 Oct;24(10):1169–1176. doi: 10.1016/0026-0495(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S., Nizet H. Renal handling of endogenous glucagon in the dog: comparison with insulin. Metabolism. 1974 Aug;23(8):753–761. doi: 10.1016/0026-0495(74)90007-9. [DOI] [PubMed] [Google Scholar]
- Maack T. Renal handling of low molecular weight proteins. Am J Med. 1975 Jan;58(1):57–64. doi: 10.1016/0002-9343(75)90533-1. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., EVERETT N. B., SIMMONS B. S., WILLIAMS R. H. Metabolism of insulin-I 131 and glucagon-I 131 in the kidney of the rat. Am J Physiol. 1958 Feb;192(2):227–231. doi: 10.1152/ajplegacy.1958.192.2.227. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence for glucagon biosynthesis involving a protein intermediate in islets of the anglerfish (Lophius americanus). Endocrinology. 1971 Sep;89(3):642–651. doi: 10.1210/endo-89-3-642. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence of sequential metabolic cleavage of proglucagon to glucagon in glucagon biosynthesis. Endocrinology. 1975 Oct;97(4):868–877. doi: 10.1210/endo-97-4-868. [DOI] [PubMed] [Google Scholar]
- Rigopoulou D., Valverde I., Marco J., Faloona G., Unger R. H. Large glucagon immunoreactivity in extracts of pancreas. J Biol Chem. 1970 Feb 10;245(3):496–501. [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H. A modified semi-automated resorcinol method for the determination of inulin. Clin Chem. 1969 Nov;15(11):1072–1078. [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. C., Fender H. R., Ramus N. I., Villar H. V., Rayford P. L. Cholecystokinin metabolism in man and dogs. Ann Surg. 1975 Oct;182(4):496–504. doi: 10.1097/00000658-197510000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakatellis A. C., Tada K., Yamaji K., Gardiki-Kouidou P. Isolation and partial characterization of anglefish proglucagon. Biochemistry. 1975 Apr 8;14(7):1508–1512. doi: 10.1021/bi00678a025. [DOI] [PubMed] [Google Scholar]
- Valverde I., Dobbs R., Unger R. H. Heterogeneity of plasma glucagon immunoreactivity in normal, depancreatized, and alloxan-diabetic dogs. Metabolism. 1975 Sep;24(9):1021–1028. doi: 10.1016/0026-0495(75)90095-5. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]


