Abstract
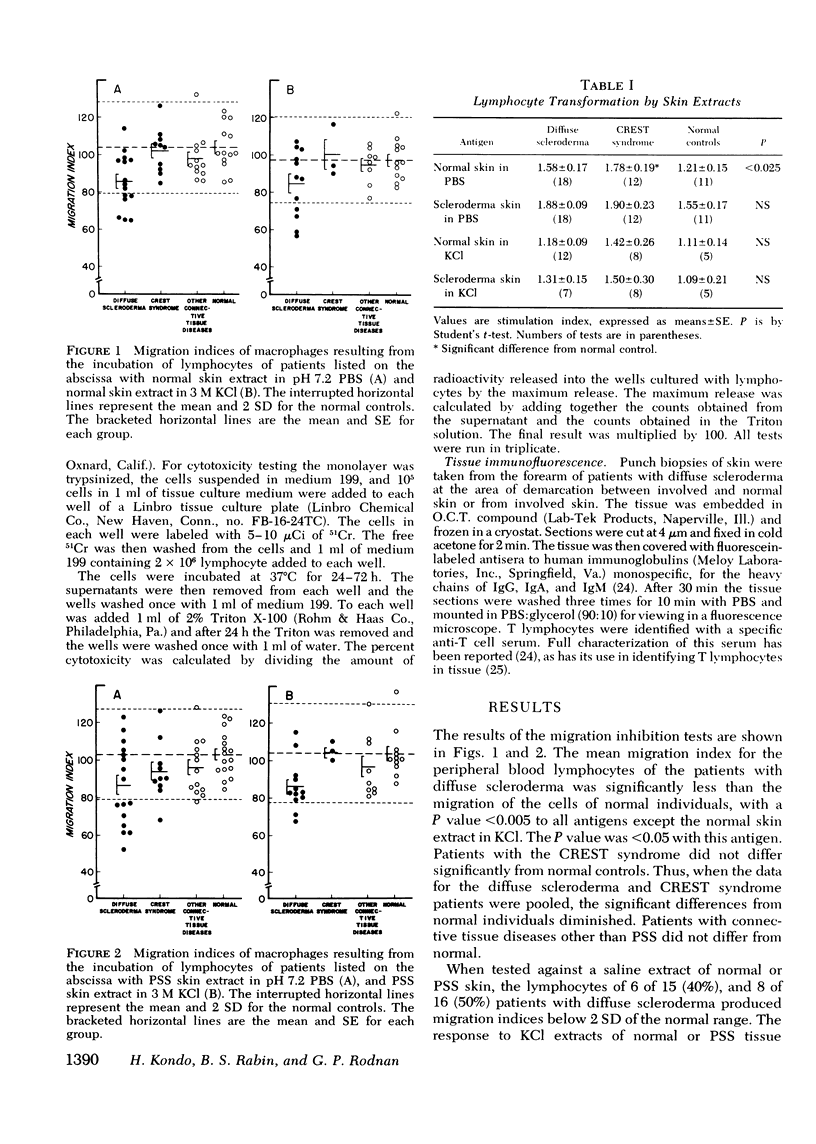
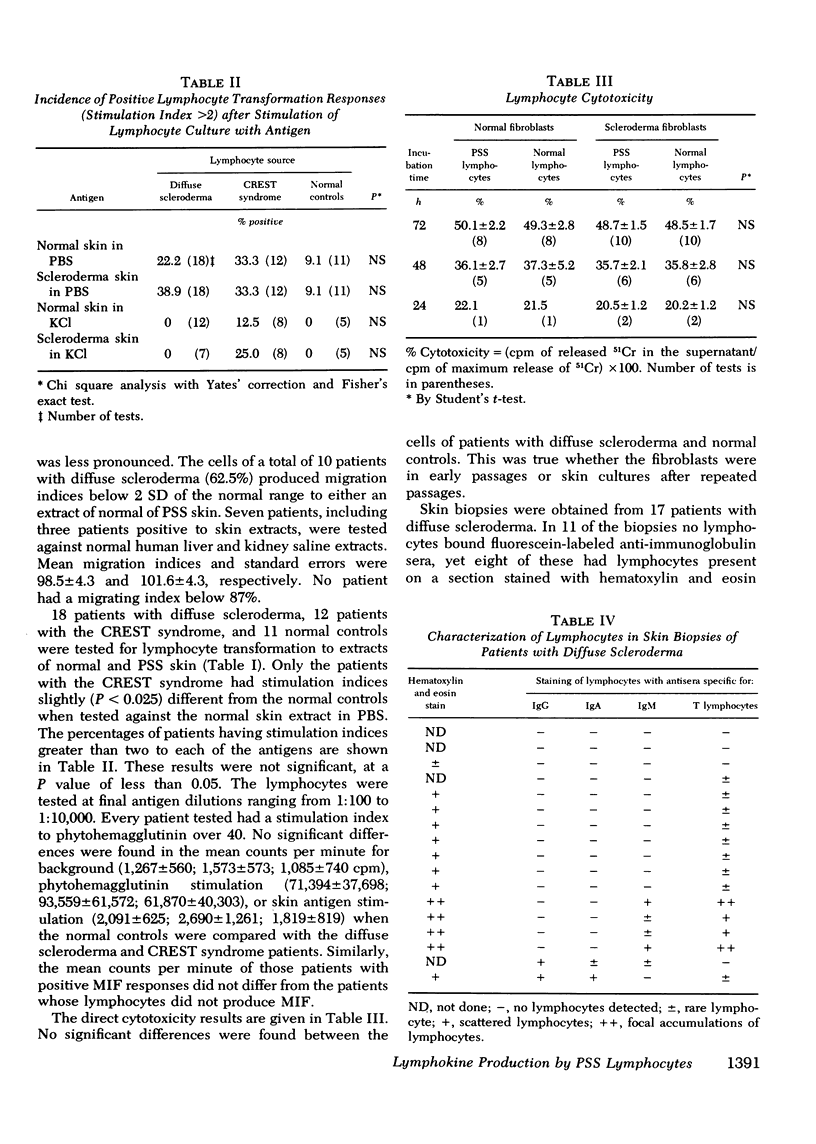
Cell-mediated immunity to skin extracts was studied by the macrophage migration inhibition test, lymphocyte transformation, and direct cytotoxicity to skin fibroblasts, in normal individuals and patients with progressive systemic sclerosis. The latter included 18 individuals with diffuse scleroderma and 12 with the CREST syndrome, a variant form of systemic sclerosis in which there is more limited involvement of the skin. Controls consisted of 13 patients with other connective tissue diseases and 16 normal individuals. Phosphate-buffered saline and 3 M KCl extracts of both normal and sclerodermatous skin were used as antigens. No evidence of lymphocyte reactivity was found by the lymphocyte transformation and direct cytotoxicity test procedures. However, the lymphocytes of patients with diffuse scleroderma did respond to extracts of both normal and sclerodermatous skin in the migration inhibition assay. 10 of 16 patients (62.5%) had migration indices below 2 SD of the normal range, 1 of 10 CREST patients and 1 of 13 patients with other connective tissue diseases showed similar reactivity. Antisera specific for immunoglobulin-bearing lymphocytes (B lymphocytes) and T lymphocytes were used to characterize the lymphocytes found in skin biopsies of patients with diffuse scleroderma. T lymphocytes made up the majority of lymphocytes in the skin infiltrates. These findings suggest that lymphocytes sensitized to skin extracts are present in patients with diffuse scleroderma. The cell-mediated immune reaction to skin antigens may be a factor in the pathogenesis of diffuse scleroderma.
Full text
PDF


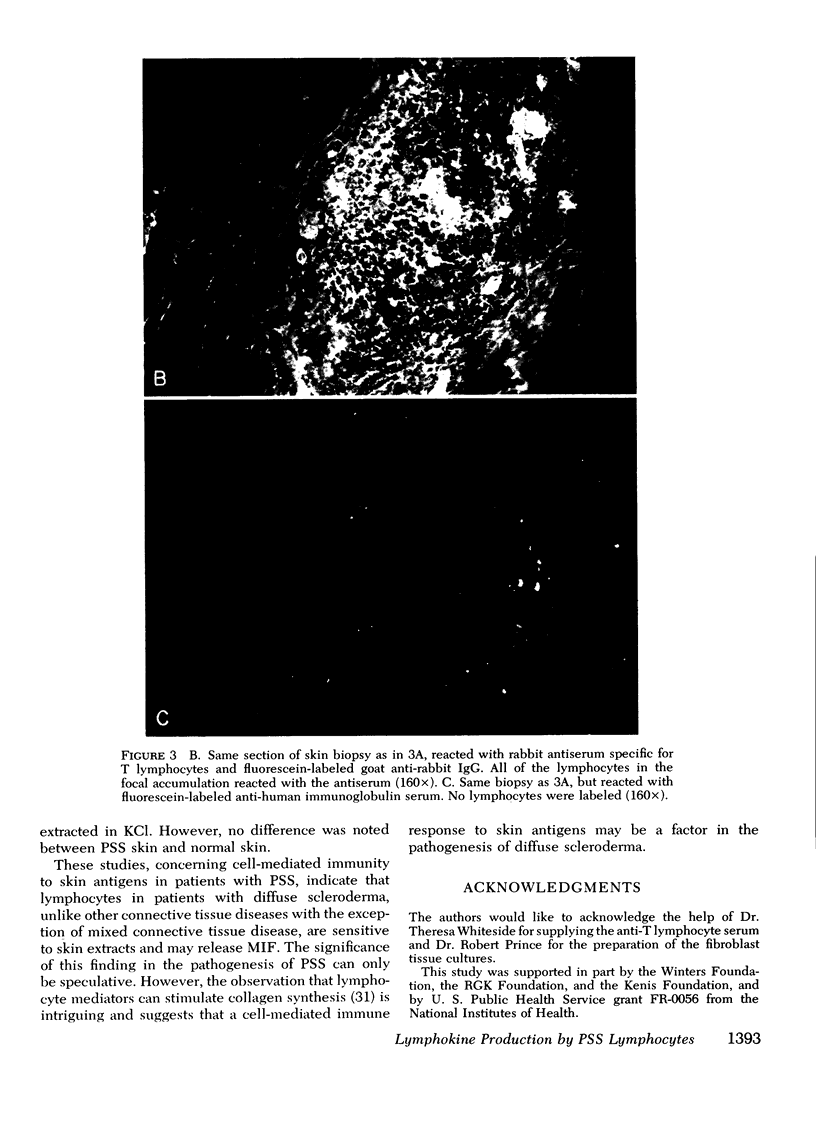

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Fishbein E. Immunochemical characterization of the anti-RNA antibodies found in scleroderma and systemic lupus erythematosus. I. Differences in reactivity with Poly (U) and Poly-(A) Poly (U). J Immunol. 1975 Jul;115(1):28–31. [PubMed] [Google Scholar]
- Alarcón-Segovia D., Ibánez G., Hernández-Ortíz J., Velázquez-Forero F., González-Jiménez Y. Sjögren's syndrome in progressive systemic sclerosis (scleroderma). Am J Med. 1974 Jul;57(1):78–85. doi: 10.1016/0002-9343(74)90771-2. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Gaffney J., Jimenez L. Dissociation of MIF production and cell proliferation. J Immunol. 1972 Dec;109(6):1395–1398. [PubMed] [Google Scholar]
- Clark J. A., Winkelmann R. K., Ward L. E. Serologic alterations in scleroderma and sclerodermatomyositis. Mayo Clin Proc. 1971 Feb;46(2):104–107. [PubMed] [Google Scholar]
- Currie S., Saunders M., Knowles M. Immunological aspects of systemic sclerosis in vitro activity of lymphocytes from patients with the disorder. Br J Dermatol. 1971 May;84(5):400–409. doi: 10.1111/j.1365-2133.1971.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Chandor S., Friou G. J., Bischel M. Progressive systemic sclerosis (PSS) and localized scleroderma (morphea) with positive LE cell test and unusual systemic manifesstations compatible with systemic lupus erythematous (SLE): presentation of 14 cases including one set of identical twins, one with scleroderma dn the other with SLE. Review of the literature. Medicine (Baltimore) 1971 May;50(3):199–222. doi: 10.1097/00005792-197105000-00003. [DOI] [PubMed] [Google Scholar]
- Eifel P. J., Walker S. M., Lucas Z. J. Standardization of a sensitive and rapid assay for lymphotoxin. Cell Immunol. 1975 Jan;15(1):208–221. doi: 10.1016/0008-8749(75)90176-8. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Damiano V., Nedwich A. Alteration of subcutaneous tissue in systemic scleroderma. Arch Dermatol. 1972 Jan;105(1):59–66. [PubMed] [Google Scholar]
- Fleischmajer R., Krol S. Chemical synthesis of the dermis in scleroderma. Proc Soc Exp Biol Med. 1967 Oct;126(1):252–256. doi: 10.3181/00379727-126-32415. [DOI] [PubMed] [Google Scholar]
- Gerber M. A. Immunohistochemical findings in the renal vascular lesions of progressive systemic sclerosis. Hum Pathol. 1975 May;6(3):343–347. doi: 10.1016/s0046-8177(75)80096-7. [DOI] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R. Leukocyte migration inhibition in progressive systemic sclerosis. Br J Dermatol. 1974 Jul;91(1):1–6. doi: 10.1111/j.1365-2133.1974.tb06709.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordon R. E., Deheer D., Schroeter A., Winkelmann R. K. Antinuclear antibodies: their significance in scleroderma. Mayo Clin Proc. 1971 Feb;46(2):111–113. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rabin B. S., Rodnan G. P., Bassion S., Gill T. J., 3rd Letter: HL-A antigens in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1975 Jul-Aug;18(4):381–382. doi: 10.1002/art.1780180418. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Kahan B. D. Biological and chemical characterization of human histocompatibility antigens. Fed Proc. 1970 Nov-Dec;29(6):2034–2040. [PubMed] [Google Scholar]
- Rothfield N. F., Rodnan G. P. Serum antinuclear antibodies in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Oct;11(5):607–617. doi: 10.1002/art.1780110502. [DOI] [PubMed] [Google Scholar]
- Senyk G., Michaeli D. Induction of cell-mediated immunity and tolerance to homologous collagen in guinea pigs: demonstration of antigen-reactive cells for a self-antigen. J Immunol. 1973 Nov;111(5):1381–1388. [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Sharp G. C. Mixed connective tissue disease. Bull Rheum Dis. 1974;25(9):828–831. [PubMed] [Google Scholar]
- TUFFANELLI D. L. Scleroderma and its relationship to the "collagenoses": dermatomyositis, lupus erythematosus, rheumatoid arthritis and Sjogren's syndrome. Am J Med Sci. 1962 Feb;243:133–146. [PubMed] [Google Scholar]
- Thor D. E., Jureziz R. E., Veach S. R., Miller E., Dray S. Cell migration inhibition factor released by antigen from human peripheral lymphocytes. Nature. 1968 Aug 17;219(5155):755–757. doi: 10.1038/219755a0. [DOI] [PubMed] [Google Scholar]
- WINTERBAUER R. H. MULTIPLE TELANGIECTASIA, RAYNAUD'S PHENOMENON, SCLERODACTYLY, AND SUBCUTANIOUS CALCINOSIS: A SYNDROME MIMICKING HEREDITARY HEMORRHAGIC TELANGIECTASIA. Bull Johns Hopkins Hosp. 1964 Jun;114:361–383. [PubMed] [Google Scholar]
- Whiteside T. L., Berardi R. S., Rabin B. S. Quantitation of human peripheral blood T and B lymphocytes. Int Arch Allergy Appl Immunol. 1975;48(6):731–738. doi: 10.1159/000231361. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Rabin B. S. Surface immunoglobulin on activated human peripheral blood thymus-derived cells. J Clin Invest. 1976 Mar;57(3):762–771. doi: 10.1172/JCI108335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein A., Rodnan G. P., Heilman J. D. Cellular immunity in progressive systemic sclerosis (scleroderma). Ann Rheum Dis. 1972 Mar;31(2):126–128. doi: 10.1136/ard.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]