Abstract
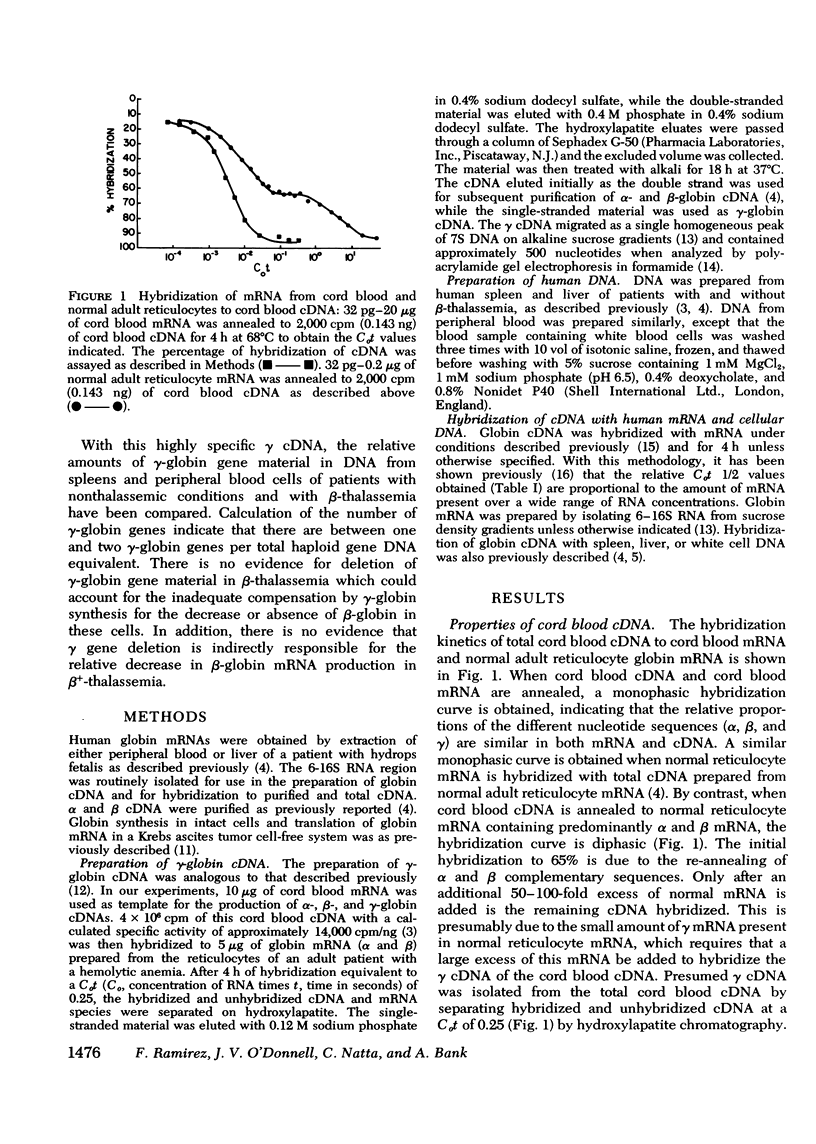
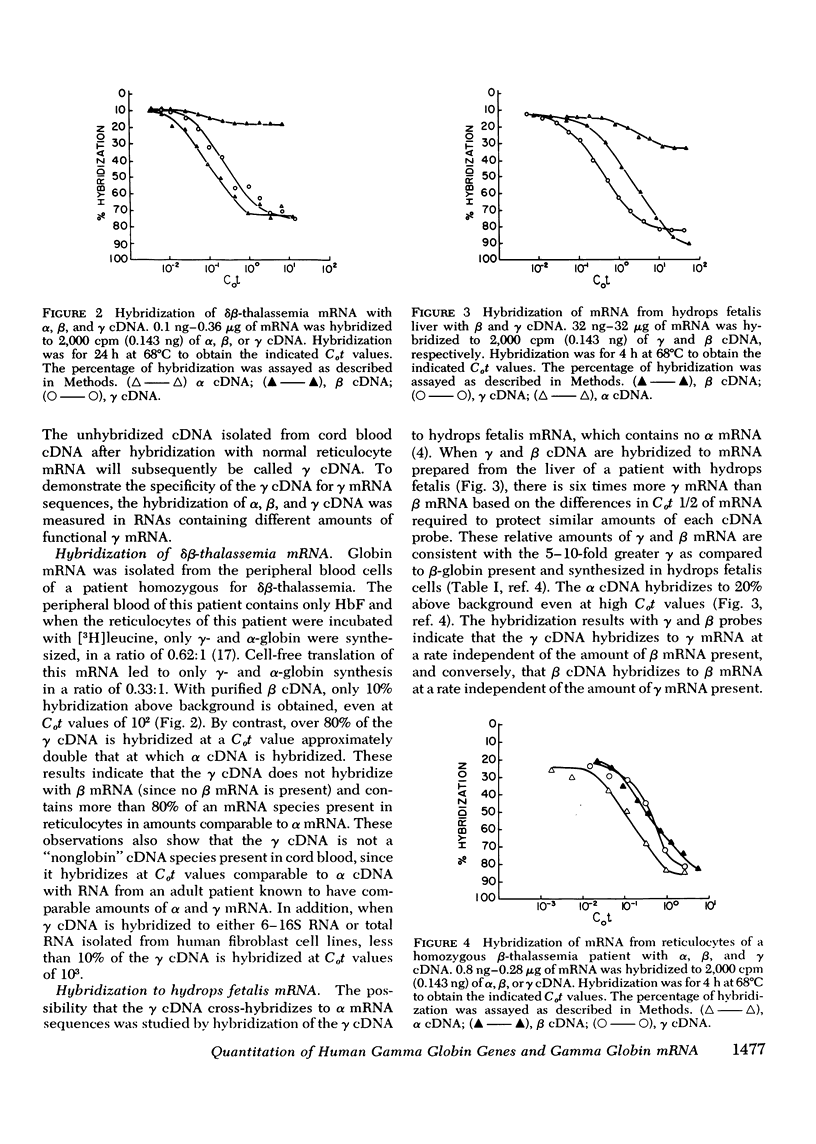
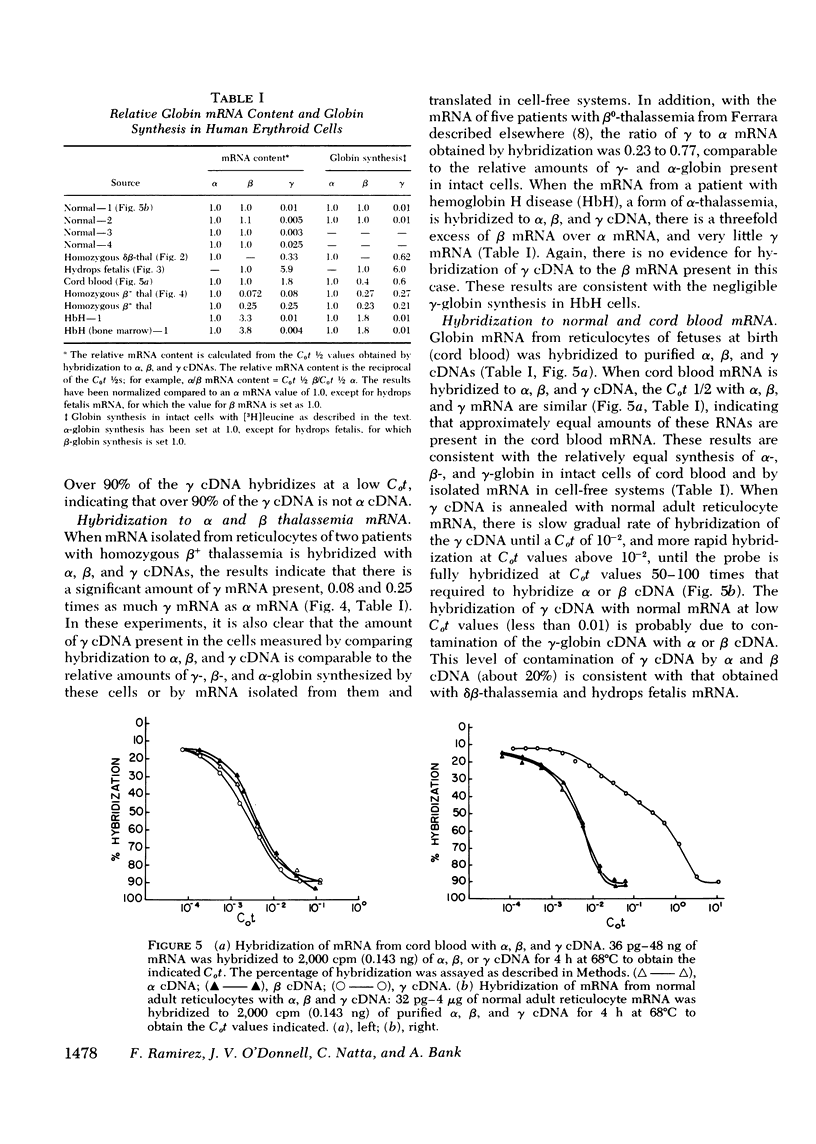
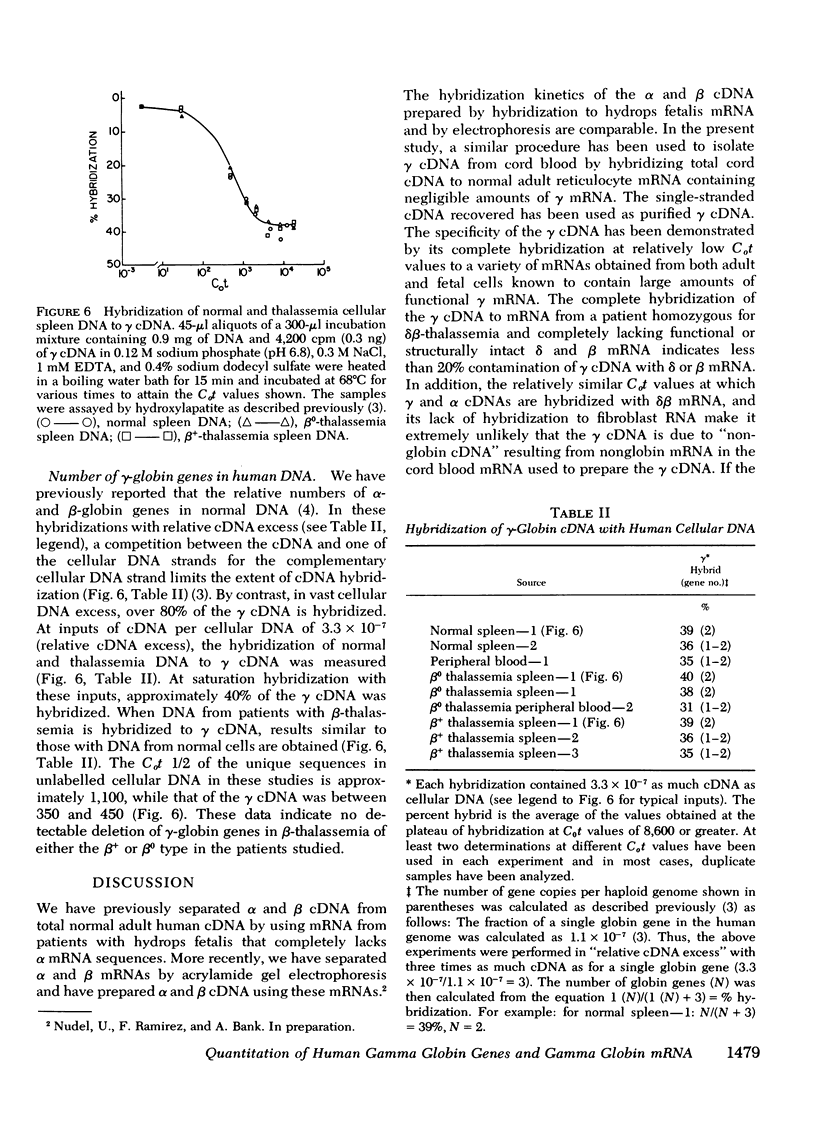
Complementary DNA (cDNA) specific for gamma-globin nucleotide sequences has been prepared by hybridizing total cDNA made from cord blood messenger RNA (mRNA) as template to an excess of normal adult human globin mRNA and recovering the single-stranded cDNA from hydroxylapatite. The specificity of the gamma cDNA for gamma mRNA sequences is strongly supported by the hybridization of this cDNA at low Cot values (Co, concentration of RNA and t, time in seconds) to RNA samples containing large amounts of functional gamma globin mRNA and the lack of hybridization to RNA samples containing little, if any, gamma-globin mRNA. The absence of cross-hybridization of gamma cDNA with alpha, beta, and delta mRNAs is demonstrated by the complete hybridization of the gamma cDNA to mRNA samples completely lacking either alpha or beta and delta mRNA. An estimate of the number of gamma-globin genes in human cellular DNA was obtained by hybridization of purified gamma cDNA to DNA from spleen and white blood cells of normal and beta-thalassemia subjects and measurement of the percent of gamma cDNA hybridized at saturation. The results indicate that there are between one and two gamma-globin genes per total haploid gene DNA equivalent obtained from both normal and beta-thalassemia subjects. These values are consistent with genetic evidence for the presence of multiple gamma gene loci in human cells. The finding that the number of gamma-globin genes in beta-thalassemia DNA is similar to that in nonthalassemia DNA indicates that a deletion of gamma-globin genes cannot account for either the inadequate gamma-globin synthesis or indirectly for the decreased or absent beta-globin synthesis in beta-thalassemia cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Reiteration frequency of duck haemoglobin genes. Nat New Biol. 1973 Feb 14;241(111):204–207. doi: 10.1038/newbio241204a0. [DOI] [PubMed] [Google Scholar]
- Dow L. W., Terada M., Natta C., Metafora S., Grossbard E., Marks P. A., Bank A. Globin synthesis of intact cells and activity of isolated mRNA in -thalassaemia. Nat New Biol. 1973 May 23;243(125):114–116. [PubMed] [Google Scholar]
- Forget B. G., Hillman D. G., Lazarus H., Barell E. F., Benz ej J. R., Caskey C. T., Huisman T. H., Schroeder W. A., Housman D. Absence of messenger RNA and gene DNA for beta-globin chains in hereditary persistence of fetal hemoglobin. Cell. 1976 Mar;7(3):323–329. doi: 10.1016/0092-8674(76)90161-6. [DOI] [PubMed] [Google Scholar]
- Gambino R., Kacian D., O'Donnell J., Ramirez F., Marks P. A., Bank A. A limited number of globin genes in human DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3966–3970. doi: 10.1073/pnas.71.10.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Gambino R., Dow L. W., Grossbard E., Natta C., Ramirez F., Spiegelman S., Marks P. A., Bank A. Decreased globin messenger RNA in thalassemia detected by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1886–1890. doi: 10.1073/pnas.70.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Charache S., Kazazian H. H. Deletion of the beta-globin structure gene in hereditary persistence of foetal haemoglobin. Nature. 1975 Nov 13;258(5531):162–163. doi: 10.1038/258162a0. [DOI] [PubMed] [Google Scholar]
- Lanyon W. G., Ottolenghi S., Williamson R. Human globin gene expression and linkage in bone marrow and fetal liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):258–262. doi: 10.1073/pnas.72.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Old J., Clegg J. B., Weatherall D. J., Ottolenghi S., Comi P., Giglioni B., Mitchell J., Tolstoshev P., Williamson R. A direct estimate of the number of human gamma-globin genes. Cell. 1976 May;8(1):13–18. doi: 10.1016/0092-8674(76)90180-x. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Lanyon W. G., Paul J., Williamson R., Weatherall D. J., Clegg J. B., Pritchard J., Pootrakul S., Boon W. H. The severe form of alpha thalassaemia is caused by a haemoglobin gene deletion. Nature. 1974 Oct 4;251(5474):389–392. doi: 10.1038/251389a0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Lanyon W. G., Williamson R., Weatherall D. J., Clegg J. B., Pitcher C. S. Human globin gene analysis for a patient with beta-o/delta beta-thalassemia. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2294–2299. doi: 10.1073/pnas.72.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packman S., Aviv H., Ross J., Leder P. A comparison of globin genes in duck reticulocytes and liver cells. Biochem Biophys Res Commun. 1972 Nov 1;49(3):813–819. doi: 10.1016/0006-291x(72)90483-4. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Gambino R., Maniatis G. M., Rifkind R. A., Marks P. A., Bank A. Changes in globin messenger RNA content during erythroid cell differentiation. J Biol Chem. 1975 Aug 10;250(15):6054–6058. [PubMed] [Google Scholar]
- Ramirez F., Natta C., O'Donnell J. V., Canale V., Bailey G., Sanguensermsri T., Maniatis G. M., Marks P. A., Bank A. Relative numbers of human globin genes assayed with purified alpha and beta complementary human DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1550–1554. doi: 10.1073/pnas.72.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Shelton J. R., Shelton J. B., Kleihauer E. F., Dozy A. M., Robberson B. Evidence for multiple structural genes for the gamma chain of human fetal hemoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):537–544. doi: 10.1073/pnas.60.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Dozy A., Kan Y. W., Varmus H. E., Lie-Injo L. E., Ganesan J., Todd D. Genetic lesion in homozygous alpha thalassaemia (hydrops fetalis). Nature. 1974 Oct 4;251(5474):392–393. doi: 10.1038/251392a0. [DOI] [PubMed] [Google Scholar]


