Abstract
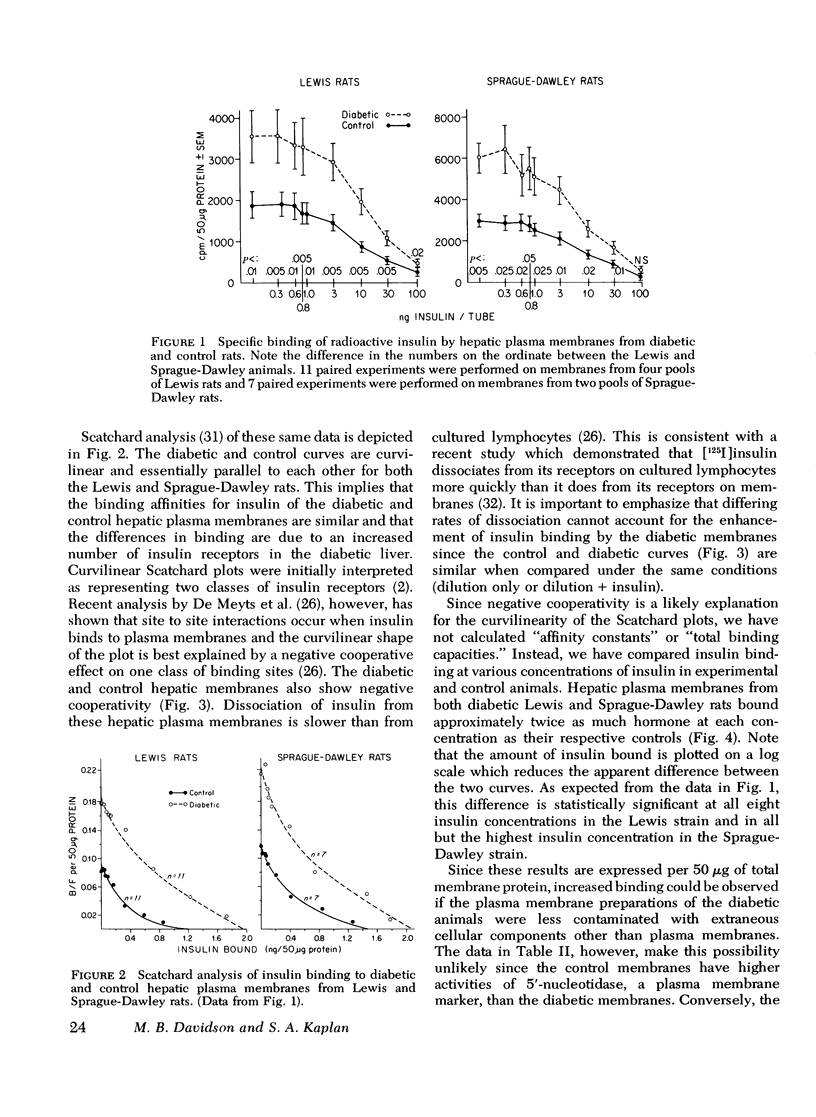
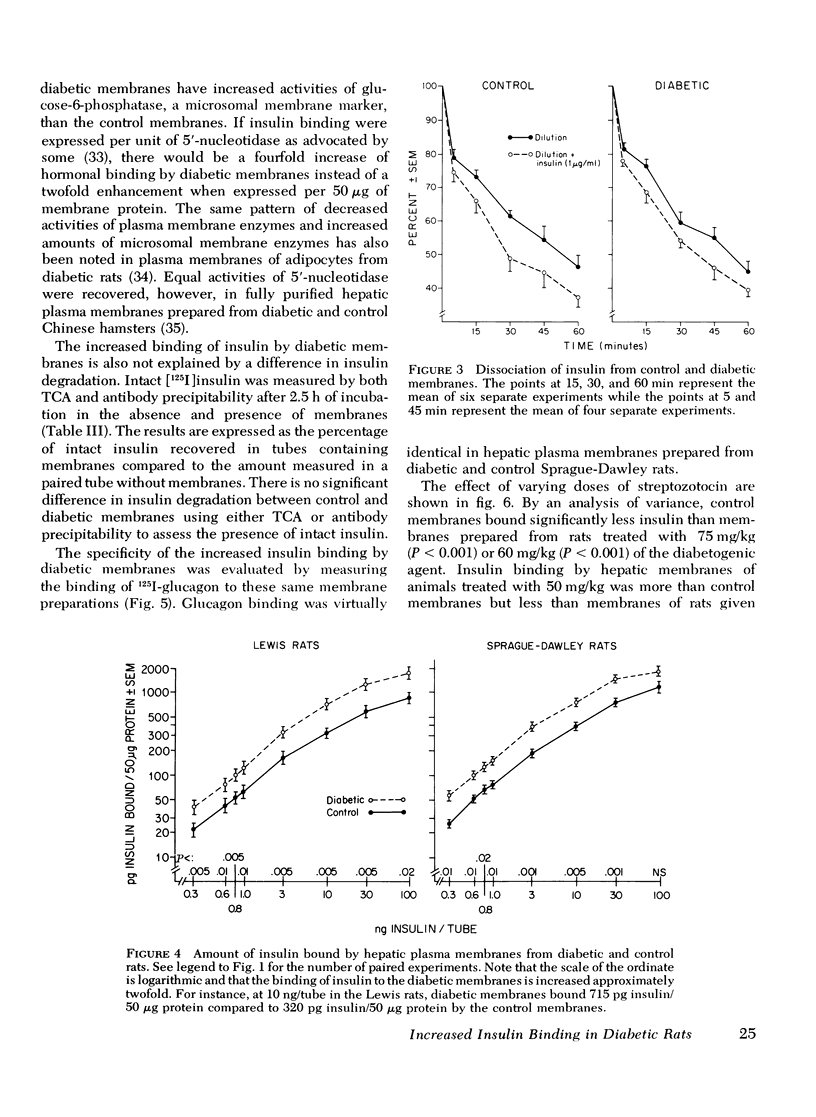
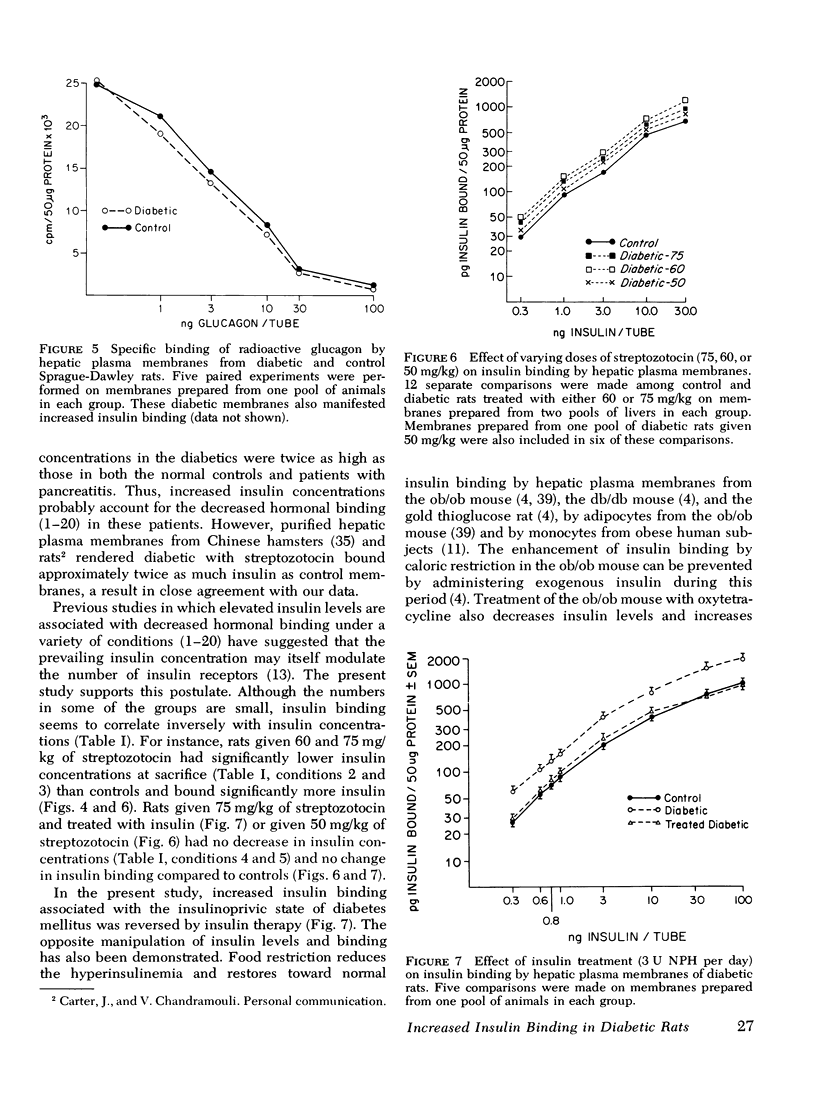
Hepatic plasma membranes prepared from rats rendered diabetic by streptozotocin bound approximately twice as much insulin per 50 mug protein as control membranes. Glucagon binding of diabetic and control membranes was virtually identical. This increased insulin binding was not due to a nonspecific effect of streptozotocin, decreased degradation of insulin slower dissociation from its receptor, or a selective higher yield of membranes prepared from the diabetic livers. Diabetic and control membranes both showed negative cooperativity. Scatchard analysis suggested that the difference in binding was due to an enhanced binding capacity of the diabetic membranes rather than increased affinity of the binding sites. Increased insulin binding of diabetic membranes was returned to normal by insulin treatment. These data are consistent with the postulate that there is an inverse relationship between circulating insulin levels and insulin binding and that insulin may modulate its own receptor. However, since it has been reported that fat, muscle, and hepatic tissue from rats made diabetic by alloxan administration are insensitive to insulin, the capacity for binding can not be the sole factor determining the response to insulin in diabetes mellitus. Therefore, sensitivity of the diabetic liver to insulin is determined, at least in part, by events subsequent to the binding of insulin to its receptor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Gavin J. R., 3rd, Lesniak M. A., Roth J. Insulin receptors in human circulating lymphocytes: application to the study of insulin resistance in man. J Clin Endocrinol Metab. 1973 Apr;36(4):627–633. doi: 10.1210/jcem-36-4-627. [DOI] [PubMed] [Google Scholar]
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D., Lazarus N. R. The control of insulin receptors in the New Zealand obese mouse. Diabetologia. 1975 Aug;11(4):261–267. doi: 10.1007/BF00422389. [DOI] [PubMed] [Google Scholar]
- Bégin-Heck N., Bourassa M., Heick H. M. The effect of oxytetracycline on insulin resistance in obese mice. Biochem J. 1974 Sep;142(3):465–475. doi: 10.1042/bj1420465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli V., Carter J. R., Jr Cell membrane changes in chronically diabetic rats. Diabetes. 1975 Mar;24(3):257–262. doi: 10.2337/diab.24.3.257. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Huang D., Cuatrecasas P. The defect in insulin receptors in obese-hyperglycemic mice: a probable accompaniment of more generalized alterations in membrane glycoproteins. Biochem Biophys Res Commun. 1975 May 19;64(2):566–573. doi: 10.1016/0006-291x(75)90359-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Feldman J. M., Lebovitz H. E. Effect of fasting on insulin secretion and action in mice. Endocrinology. 1970 Feb;86(2):313–321. doi: 10.1210/endo-86-2-313. [DOI] [PubMed] [Google Scholar]
- Forgue M. E., Freychet P. Insulin receptors in the heart muscle. Demonstration of specific binding sites and impairment of insulin binding in the plasma membrane of the obese hyperglycemic mouse. Diabetes. 1975 Aug;24(8):715–723. doi: 10.2337/diab.24.8.715. [DOI] [PubMed] [Google Scholar]
- Freeman C., Karoly K., Adelman R. C. Impairments in availability of insulin to liver in vivo and in binding of insulin to purified hepatic plasma membrane during aging. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1573–1580. doi: 10.1016/0006-291x(73)91166-2. [DOI] [PubMed] [Google Scholar]
- Freychet P. Interactions polypeptide hormones with cell membrane specific receptors: studies with insulin and glucagon. Diabetologia. 1976 May;12(2):83–100. doi: 10.1007/BF00428972. [DOI] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Freychet P., Laudat M. H., Laudat P., Rosselin G., Kahn C. R., Gorden P., Roth J. Impairment of insulin binding to the fat cell plasma membrane in the obese hyperglycemic mouse. FEBS Lett. 1972 Sep 15;25(2):339–342. doi: 10.1016/0014-5793(72)80519-2. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975 May 10;250(9):3368–3374. [PubMed] [Google Scholar]
- Goldfine I. D. Binding of insulin to thymocytes from suckling and hypophysectomized rats: evidence for two mechanisms regulating insulin sensitivity. Endocrinology. 1975 Oct;97(4):948–954. doi: 10.1210/endo-97-4-948. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Kahn C. R., Neville D. M., Jr, Roth J., Garrison M. M., Bates R. W. Decreased binding of insulin to its receptors in rats with hormone induced insulin resistance. Biochem Biophys Res Commun. 1973 Aug 6;53(3):852–857. doi: 10.1016/0006-291x(73)90171-x. [DOI] [PubMed] [Google Scholar]
- Haft D. E. Studies of the metabolism of isolated livers of normal and alloxan-diabetic rats perfused with insulin. Diabetes. 1968 May;17(5):244–250. doi: 10.2337/diab.17.5.244. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Langley J., Funcke HJ Von, Renner R., Kemmler W. Increased insulin binding capacity of liver membranes from diabetic Chinese hamsters. Nature. 1975 Nov 13;258(5531):154–154. doi: 10.1038/258154a0. [DOI] [PubMed] [Google Scholar]
- Huang D., Cuatrecasas P. Insulin-induced reduction of membrane receptor concentrations in isolated fat cells and lymphocytes. Independence from receptor occupation and possible relation to proteolytic activity of insulin. J Biol Chem. 1975 Oct 25;250(20):8251–8259. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Gorden P., Freychet P., Roth J. Insulin receptor defect in insulin resistance: studies in the obese-hyperglycimic mouse. Biochem Biophys Res Commun. 1972 Jul 11;48(1):135–142. doi: 10.1016/0006-291x(72)90354-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Johnson J., Liu F., Jen P., Reaven G. M. The effects of acute and chronic dexamethasone administration on insulin binding to isolated rat hepatocytes and adipocytes. Metabolism. 1975 Apr;24(4):517–527. doi: 10.1016/0026-0495(75)90076-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Decreased insulin binding to lymphocytes from diabetic subjects. J Clin Invest. 1974 Dec;54(6):1323–1328. doi: 10.1172/JCI107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J., Bacon V. C., Baur S. Insulin receptors of skeletal muscle: specific insulin binding sites and demonstration of decreased numbers of sites in obese rats. Metabolism. 1976 Feb;25(2):179–191. doi: 10.1016/0026-0495(76)90048-2. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Prolactin receptors in rat liver: possible induction by prolactin. Science. 1975 Apr 4;188(4183):57–59. doi: 10.1126/science.163493. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Kahn C. R., Neville D. M., Jr Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J Biol Chem. 1975 Jun 25;250(12):4702–4707. [PubMed] [Google Scholar]
- Vann Bennett G., Cuatrecasas P. Insulin receptor of fat cells in insulin-resistant metabolic states. Science. 1972 May 19;176(4036):805–806. doi: 10.1126/science.176.4036.805. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]



