Abstract
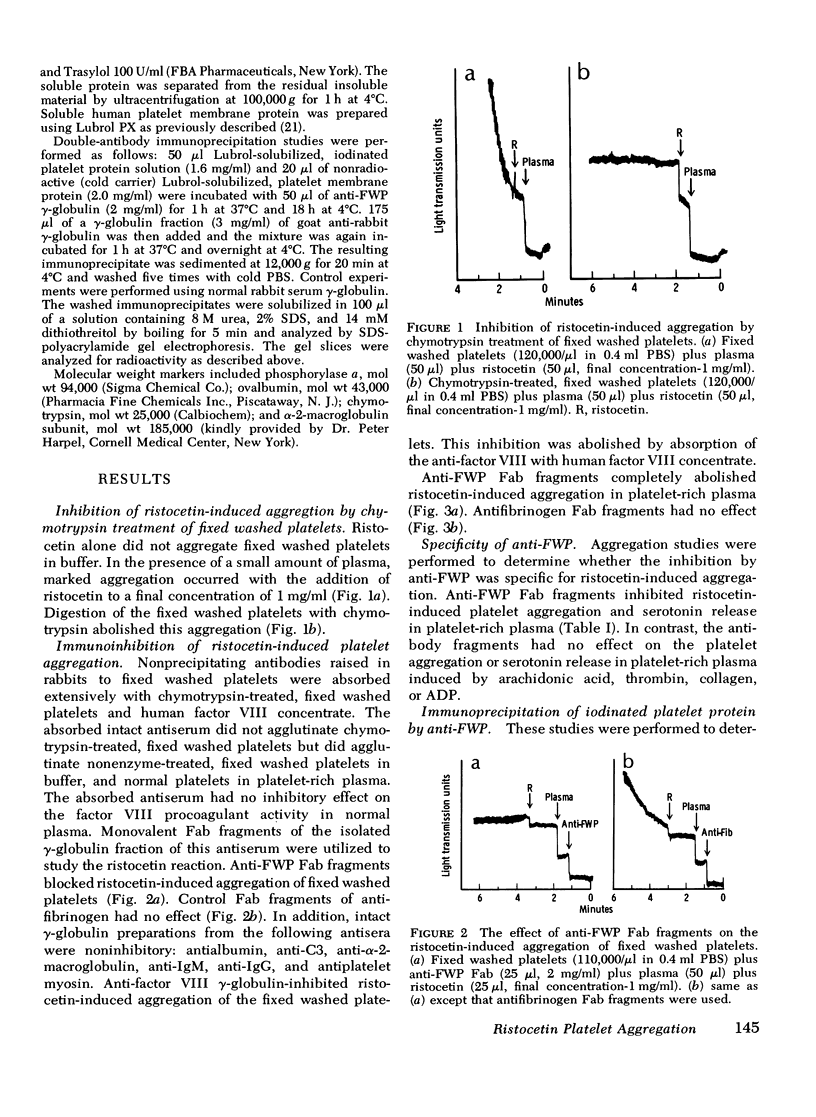
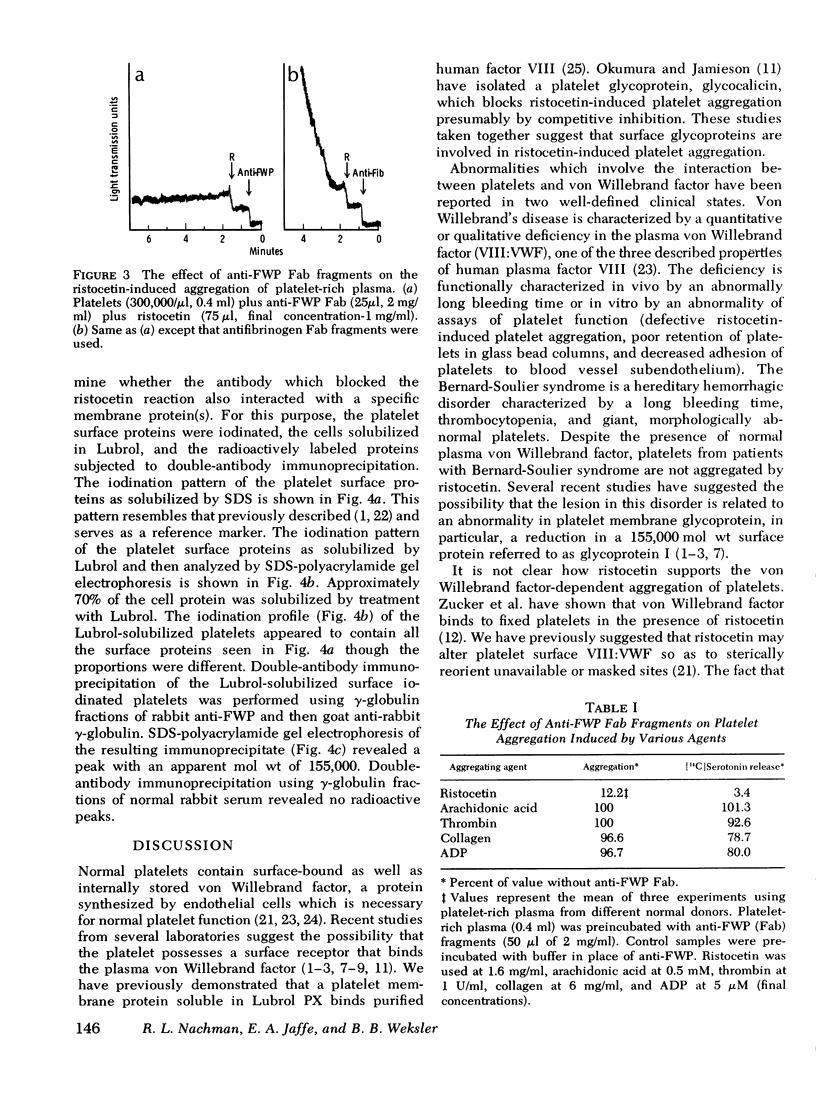
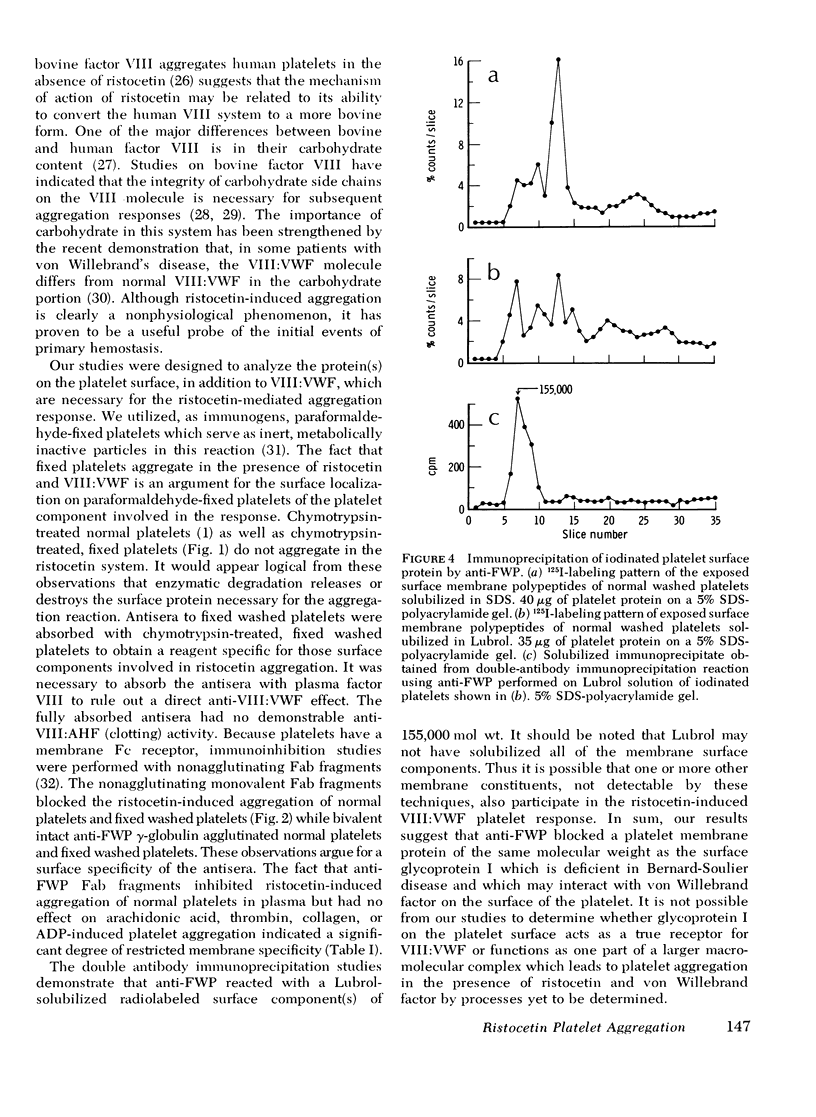
Human platelets washed and fixed in paraformaldehyde aggregate in the presence of the antibiotic ristocetin and normal plasma. This aggregation response is abolished after digestion of the fixed platelets with chymotrypsin. Antisera to fixed washed platelets were produced in rabbits and absorbed with chymotrypsin-treated, fixed washed platelets. Monovalent Fab fragments obtained from the isolated gamma-globulin fractions of the antisera blocked ristocetin-induced aggregation of fixed washed platelets in buffer and normal platelets in platelet-rich plasma. By double-antibody immunoprecipitation, it was shown that the antibody which blocked the ristocetin reaction interacted with a platelet membrane surface protein of mol wt 155,000. The results suggest that the glycoprotein I complex on the surface of the human platelet mediates ristocetin-induced von Willebrand factor-dependent platelet aggregation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- BRECKENRIDGE R. T., RATNOFF C. D. Studies on the nature of the circulating anticoagulant directed against antihemophilic factor: with notes on an assay for anthemophilic factor. Blood. 1962 Aug;20:137–149. [PubMed] [Google Scholar]
- Becker C. G., Nachman R. L. Contractile proteins of endothelial cells, platelets and smooth muscle. Am J Pathol. 1973 Apr;71(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Caen J. P., Nurden A. T., Jeanneau C., Michel H., Tobelem G., Levy-Toledano S., Sultan Y., Valensi F., Bernard J. Bernard-Soulier syndrome: a new platelet glycoprotein abnormality. Its relationship with platelet adhesion to subendothelium and with the factor VIII von Willebrand protein. J Lab Clin Med. 1976 Apr;87(4):586–596. [PubMed] [Google Scholar]
- Gralnick H. R., Coller B. S., Sultan Y. Carbohydrate deficiency of the factor VIII/von Willebrand factor Protein in von Willebrand's disease variants. Science. 1976 Apr 2;192(4234):56–59. doi: 10.1126/science.1083071. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Spiegelberg H. L. Release of serotonin from human platelets induced by aggregated immunoglobulins of different classes and subclasses. J Clin Invest. 1973 May;52(5):1282–1288. doi: 10.1172/JCI107296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. A., Hutton R. A., Hardisty R. M. Hereditary giant platelet syndrome: a disorder of a new aspect of platelet function. Br Med J. 1973 Jun 9;2(5866):586–588. doi: 10.1136/bmj.2.5866.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. A., Montgomery D. C., Hardisty R. M. Factor-VIII-related antigen in platelets. Thromb Res. 1974 May;4(5):617–624. doi: 10.1016/0049-3848(74)90218-7. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C. S., Phillips D. R., Clemetson K. J., Meyer D., Larrieu M. J., Lüscher E. F. Platelet membrane glycoproteins implicated in ristocetin-induced aggregation. Studies of the proteins on platelets from patients with Bernard-Soulier syndrome and von Willebrand's disease. J Clin Invest. 1976 Jan;57(1):112–124. doi: 10.1172/JCI108251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Mills D. C. The interaction of bovine factor VIII with human platelets. J Clin Invest. 1975 Aug;56(2):491–502. doi: 10.1172/JCI108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Haxby J. A., Arroyave C. M., Müller-Eberhard H. J. Molecular analysis of the membrane attack mechanism of complement. J Exp Med. 1972 Mar 1;135(3):549–566. doi: 10.1084/jem.135.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legaz M. E., Schmer G., Counts R. B., Davie E. W. Isolation and characterization of human Factor VIII (antihemophilic factor). J Biol Chem. 1973 Jun 10;248(11):3946–3955. [PubMed] [Google Scholar]
- Levy-Toledano S., Caen J. P., Halmos T., Mester L. Dissociation between human platelet agglomerating activity and factor VIII procoagulant activity of bovine plasma preparations by chemical treatment. I. Effect of neuraminidase. Pathol Biol (Paris) 1973 Nov;21(Suppl):60–62. [PubMed] [Google Scholar]
- Mello Périssé A. C., Soria J., Soria C., Mester L. Dissociation between human platelet agglomerating activity and factor VIII procoagulant activity of bovine plasma preparations by chemical treatment. II. Effect of periodate oxidation. Pathol Biol (Paris) 1973 Nov;21(Suppl):63–65. [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Binding of adenosine diphosphate by isolated membranes from human platelets. J Biol Chem. 1974 Feb 10;249(3):704–710. [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Studies on the proteins of human platelet membranes. J Biol Chem. 1972 Jul 25;247(14):4468–4475. [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- Nachman R. L., Jaffe E. A. Subcellular platelet factor VIII antigen and von Willebrand factor. J Exp Med. 1975 May 1;141(5):1101–1113. doi: 10.1084/jem.141.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Marcus A. J., Safier L. B. Platelet thrombosthenin: subcellular localization and function. J Clin Invest. 1967 Aug;46(8):1380–1389. doi: 10.1172/JCI105630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974 Oct;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Role of surface glycoproteins in human platelet function. Thromb Haemost. 1976 Feb 29;35(1):139–150. [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975 Jun 26;255(5511):720–722. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- Okumura T., Jamieson G. A. Platelet glycocalicin: a single receptor for platelet aggregation induced by thrombin or ristocetin. Thromb Res. 1976 May;8(5):701–706. doi: 10.1016/0049-3848(76)90250-4. [DOI] [PubMed] [Google Scholar]
- Phillips D. R. Effect of trypsin on the exposed polypeptides and glycoproteins in the human platelet membrane. Biochemistry. 1972 Nov 21;11(24):4582–4588. doi: 10.1021/bi00774a025. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jenkins C. S., Lüscher E. F., Larrieu M. Molecular differences of exposed surface proteins on thrombasthenic platelet plasma membranes. Nature. 1975 Oct 16;257(5527):599–600. doi: 10.1038/257599a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Thomas S. M., Niederman R. Human platelet myosin. I. Purification by a rapid method applicable to other nonmuscle cells. Anal Biochem. 1974 Jul;60(1):258–266. doi: 10.1016/0003-2697(74)90152-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss H. J., Hoyer L. W., Rickles F. R., Varma A., Rogers J. Quantitative assay of a plasma factor deficient in von Willebrand's disease that is necessary for platelet aggregation. Relationship to factor VIII procoagulant activity and antigen content. J Clin Invest. 1973 Nov;52(11):2708–2716. doi: 10.1172/JCI107465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]


