Abstract
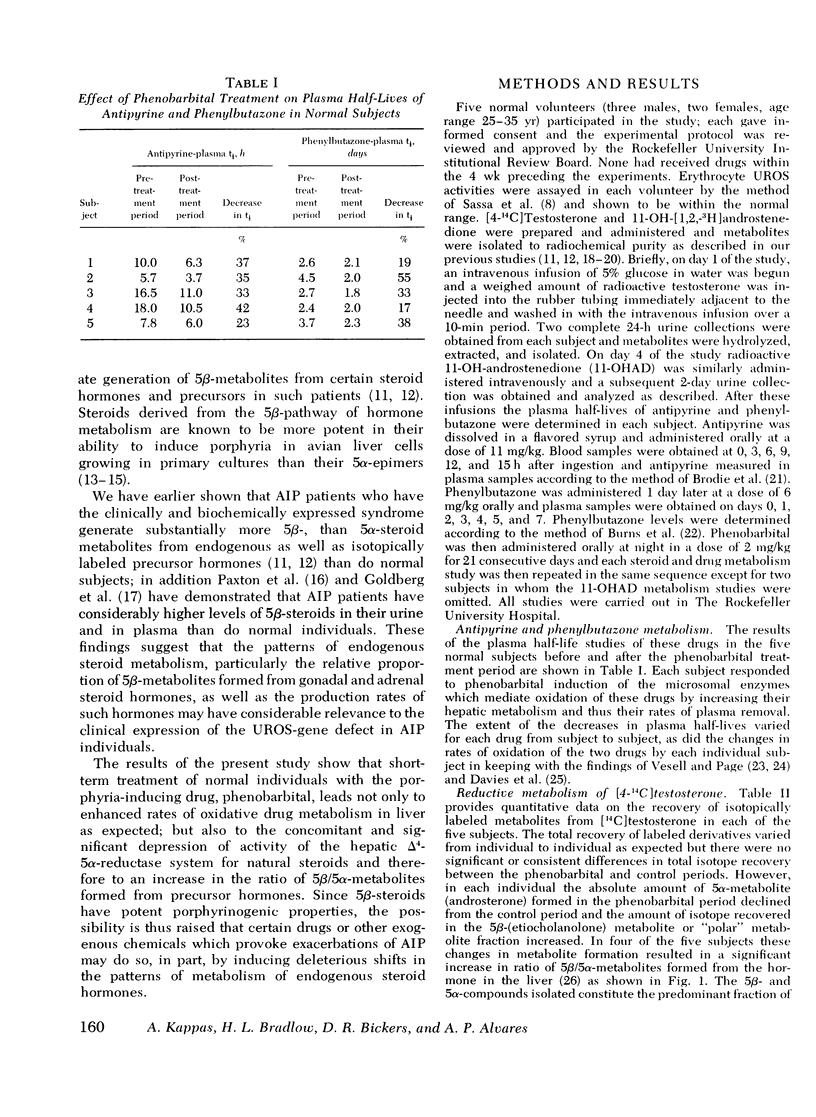
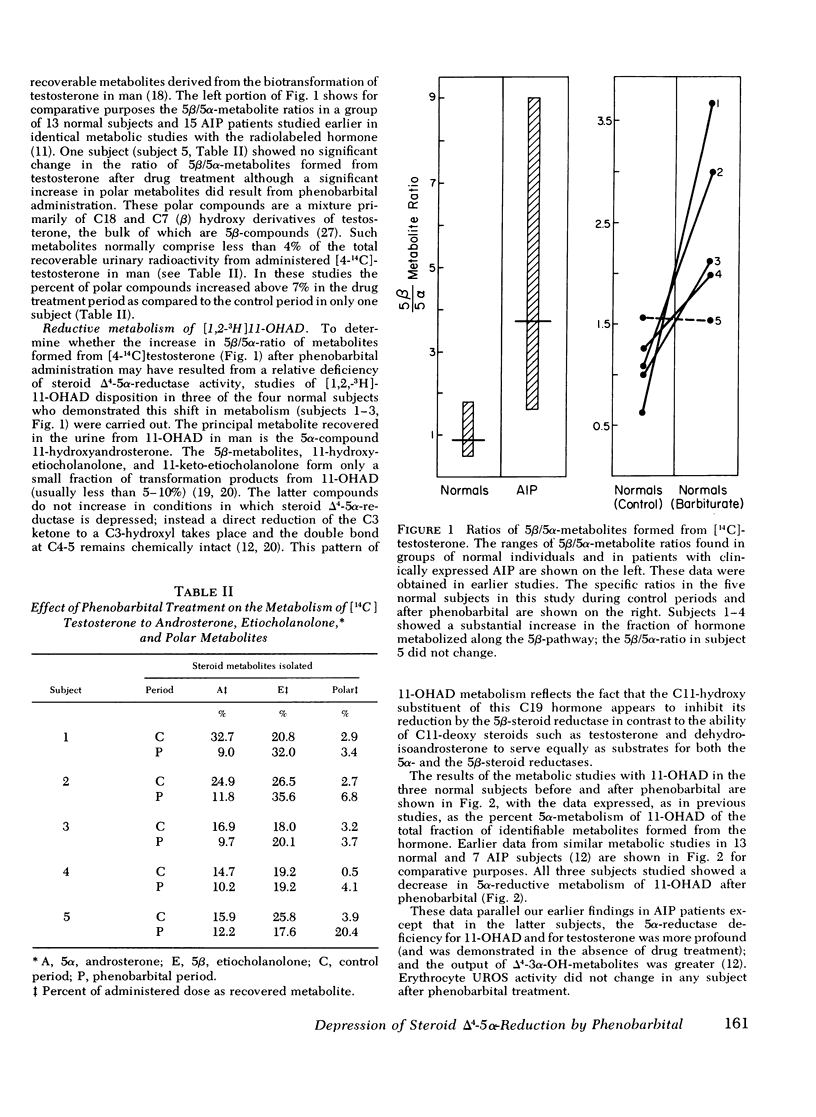
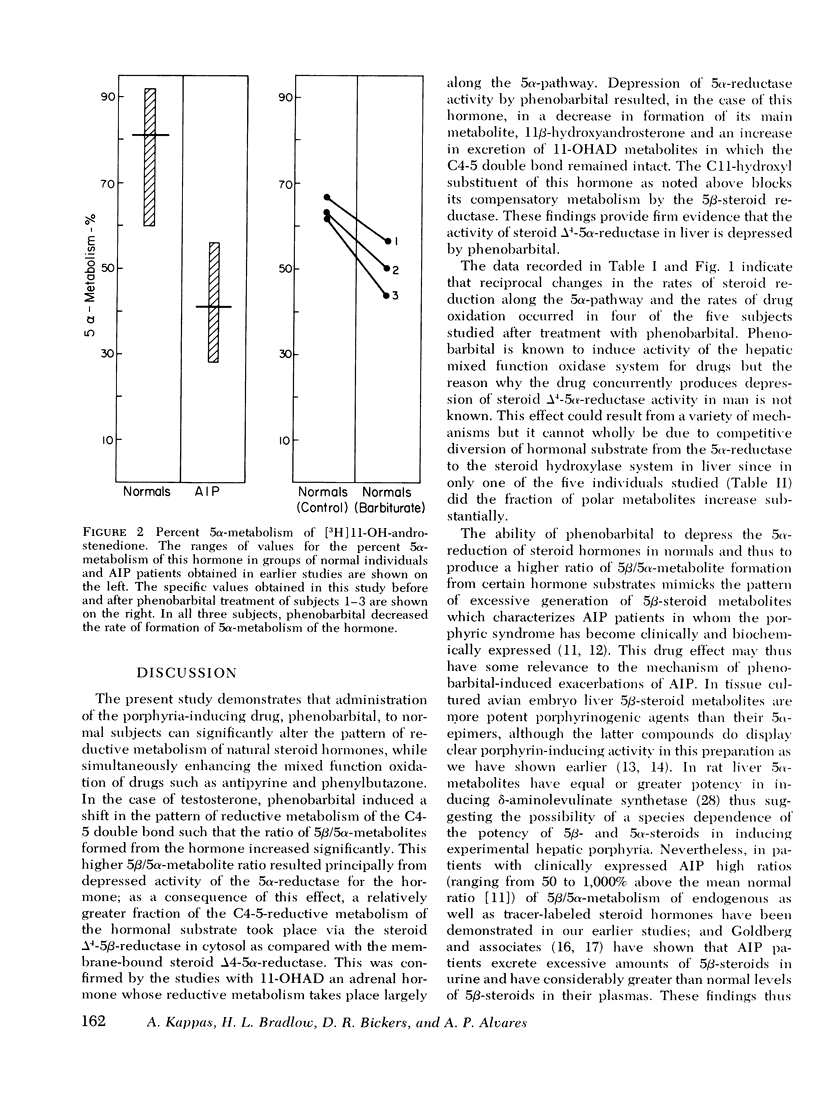
The hepatic enzymes that catalyze drug oxidations and the reductive metabolism of steroid hormones to 5alpha-derivatives are localized in membranes of the endoplasmic reticulum. Phenobarbital, which exacerbates acute intermittent porphyria in man, induces drug-oxidizing enzymes in liver. Additionally, patients in whome the primary gene defect (uroporphyrinogen-I-synthetase deficiency) of acute intermittent porphyria has become clinically expressed have low levels of hepatic steroid delta4-5alpha-reductase activity. This 5alpha-reductase deficiency in acute intermittent porphyria leads to the disproportionate generation of 5beta-steroid metabolites from precursor hormones; such steroid metabolites have significant porphyria-inducing action experimentally. In this study the effects of phenobarbital on drug oxidation and steroid 5alpha-reduction in man were examined to determine if this drug could produce changes in steroid 5alpha-reductase activity which mimicked those seen in patients with acute intermittent porphyria. Metabolic studies with [14C]-testosterone and 11beta-[3H]hydroxyandrostenedione were carried out in five normal volunteers. In all five subjects phenobarbital administration (2 mg/kg/per day for 21 days) enhanced plasma removal of the test drugs antipyrine and phenylbutazone as expected; but in four subjects phenobarbital also substantially depressed 5alpha-metabolite formation from [14C]testosterone and resulted in a pattern of hormone biotransformation characterized by a high ratio of 5beta/5alpha-metabolite formation. Studies with 11beta-[3H]hydroxy-androstenedione in three subjects confirmed that phenobarbital produced this high 5beta/5alpha ratio of steroid metabolism by depressing 5alpha-reductase activity for steroid hormones in liver. The high ratio of 5beta/5alpha-metabolites formed in normals after drug treatment mimicks the high 5beta/5alpha-steroid metabolite ratio formed from endogenous hormones in acute intermittent porphyria. The proximate mechanism by which phenobarbital induces reciprocal changes in activities of the microsomal enzymes which catalyze drug oxidations and steroid 5alpha-reductions is not known. This action of phenobarbital raises the possibility, however, that certain drugs which provoke exacerbations of human porphyria may do so, in part, by producing deleterious shifts in the patterns of endogenous steroid hormone metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLOW H. L., GALLAGHER T. F. Metabolism of 11 beta-hydroxy-delta 4-androstene-3,17-dione in man. J Biol Chem. 1957 Nov;229(1):505–518. [PubMed] [Google Scholar]
- BURNS J. J., ROSE R. K., CHENKIN T., GOLDMAN A., SCHULERT A., BRODIE B. B. The physiological disposition of phenylbutazone (butazolidin) in man and a method for its estimation in biological material. J Pharmacol Exp Ther. 1953 Nov;109(3):346–357. [PubMed] [Google Scholar]
- Bardin C. W., Mahoudeau J. A. Dynamics of androgen metabolism in women with hirsutism. Ann Clin Res. 1970 Dec;2(4):251–262. [PubMed] [Google Scholar]
- Bradlow H. L., Gillette P. N., Gallagher T. F., Kappas A. Studies in porphyria. II. Evidence for a deficiency of steroid delta-4-5-alpha-reductase activity in acute intermittent porphyria. J Exp Med. 1973 Oct 1;138(4):754–763. doi: 10.1084/jem.138.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow H. L., Zumoff B., Fukushima D. K., Hellman L., Gallagher T. F. Metabolism of 11 beta-hydroxy-delta 4-androstene-3,17-dione: effect of thyroid hormone. J Clin Endocrinol Metab. 1966 Sep;26(9):949–954. doi: 10.1210/jcem-26-9-949. [DOI] [PubMed] [Google Scholar]
- CUCINELL S. A., CONNEY A. H., SANSUR M., BURNS J. J. DRUG INTERACTIONS IN MAN. I. LOWERING EFFECT OF PHENOBARBITAL ON PLASMA LEVELS OF BISHYDROXYCOUMARIN (DICUMAROL) AND DIPHENYLHYDANTOIN (DILANTIN). Clin Pharmacol Ther. 1965 Jul-Aug;6:420–429. doi: 10.1002/cpt196564420. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Davies D. S., Thorgeirsson S. S., Breckenridge A., Orme M. Interindividual differences in rates of drug oxidation in man. Drug Metab Dispos. 1973 Jan-Feb;1(1):411–417. [PubMed] [Google Scholar]
- Edwards A. M., Elliott W. H. Induction of delta-aminolevulinic acid synthetase in isolated rat liver cells by steroids. J Biol Chem. 1975 Apr 10;250(7):2750–2755. [PubMed] [Google Scholar]
- FUKUSHIMA D. K., BRADLOW H. L., HELLMANL, GALLAGHER T. F. Isolation and characterization of 18-hydroxy-17-ketosteroids. J Biol Chem. 1962 Nov;237:3359–3363. [PubMed] [Google Scholar]
- Goldberg A., Moore M. R., Beattie A. D., Hall P. E., McCallum J., Grant J. K. Excessive urinary excretion of certain porphyrinogenic steroids in human acute intermittent porphyria. Lancet. 1969 Jan 18;1(7586):115–118. doi: 10.1016/s0140-6736(69)91134-9. [DOI] [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem. 1967 Oct 25;242(20):4587–4593. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Studies in porphyria. I. A defect in the reductive transformation of natural steroid hormones in the hereditary liver disease, acute intermittent porphyria. J Exp Med. 1972 Nov 1;136(5):1043–1053. doi: 10.1084/jem.136.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Levere R. D., Gallagher T. F. A defect of steroid hormone metabolism in acute intermittent porphyria. Fed Proc. 1972 Jul-Aug;31(4):1293–1297. [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- Kappas A., Sassa S., Granick S., Bradlow H. L. Endocrine-gene interaction in the pathogenesis of acute intermittent porphyria. Res Publ Assoc Res Nerv Ment Dis. 1974;53:225–237. [PubMed] [Google Scholar]
- Kappas A., Song C. S., Levere R. D., Sachson R. A., Granick S. THE INDUCTION OF delta-AMINOLEVULINIC ACID SYNTHETASE in vivo IN CHICK EMBRYO LIVER BY NATURAL STEROIDS. Proc Natl Acad Sci U S A. 1968 Oct;61(2):509–513. doi: 10.1073/pnas.61.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Miyagi K., Cardinal R., Bossenmaier I., Watson C. J. The serum porphobilinogen and hepatic porphobilinogen deaminase in normal and porphyric individuals. J Lab Clin Med. 1971 Nov;78(5):683–695. [PubMed] [Google Scholar]
- Paxton J. W., Moore M. R., Deattie A. D., Goldberg A. 17-Oxosteroid conjugates in plasma and urine of patients with acute intermittent porphyria. Clin Sci Mol Med. 1974 Feb;46(2):207–222. doi: 10.1042/cs0460207. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of drug levels in man: antipyrine. Science. 1968 Jul 5;161(3836):72–73. doi: 10.1126/science.161.3836.72. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of drug levels in man: phenylbutazone. Science. 1968 Mar 29;159(3822):1479–1480. doi: 10.1126/science.159.3822.1479. [DOI] [PubMed] [Google Scholar]


