Abstract
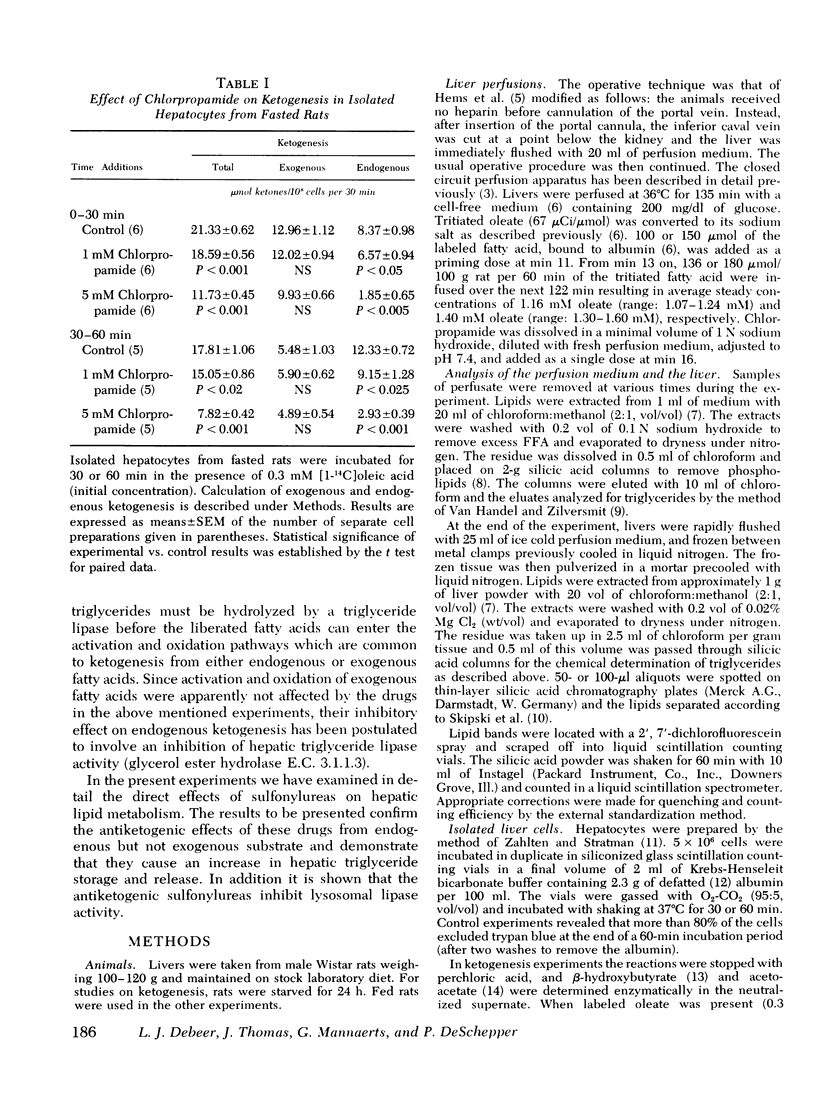
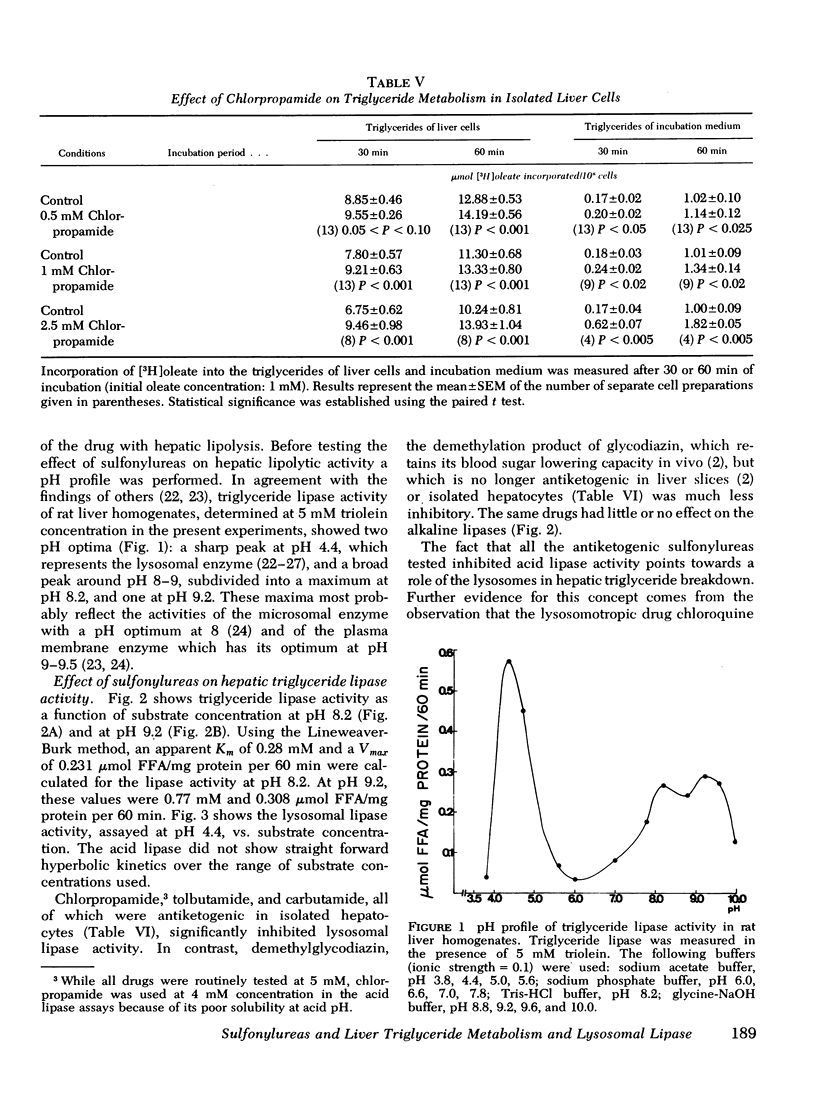
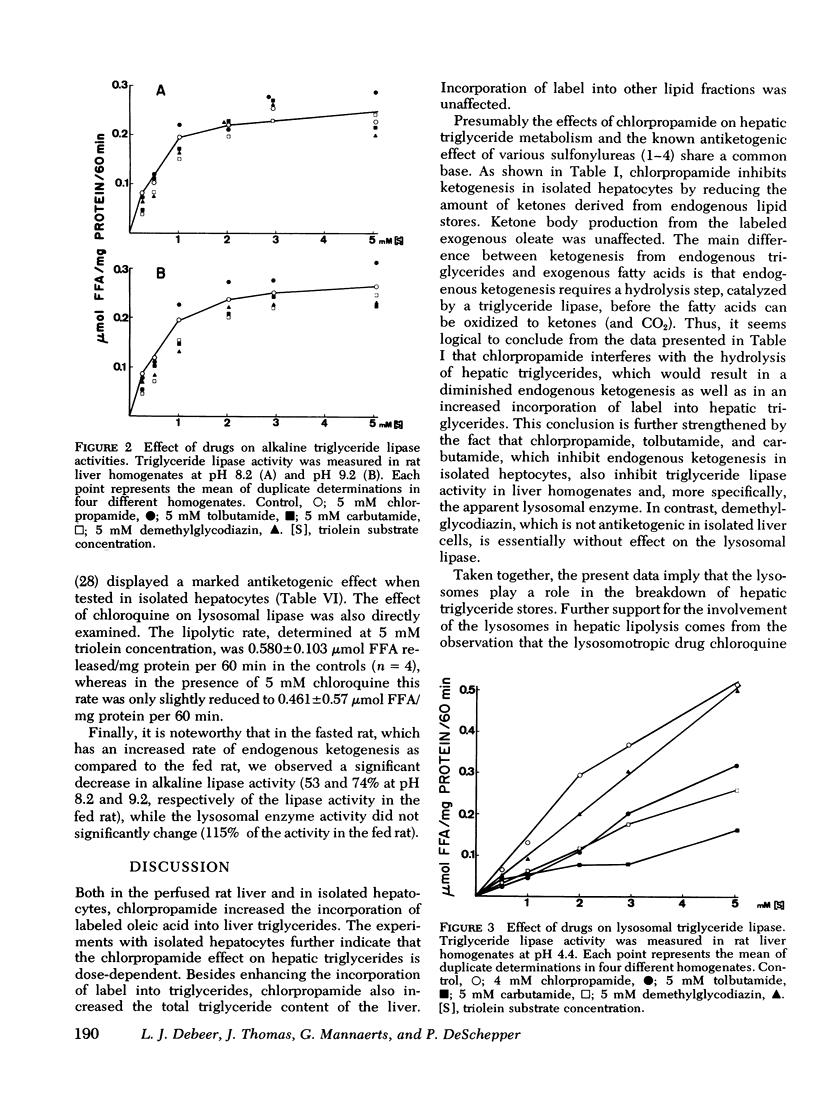
It has been suggested previously that chlorpropamide and other hypoglycemic sulfonylureas interfere with hepatic triglyceride breakdown. Since ketogenesis from endogenous hepatic lipid stores is a measure of hepatic triglyceride hydrolysis, ketogenesis derived from endogenous lipids as well as ketogenesis derived from exogenously added isotopic oleate was determined in isolated hepatocytes from fasted rats in an attempt to identify the nature of the direct effects of sulfonylureas on hepatic lipid metabolism. Ketogenesis from endogenous lipids was inhibited by 1 mM chlorpropamide, while ketone production from exogenous oleate did not change. The effect of chlorpropamide on hepatic triglyceride metabolism was further studied in the isolated perfused liver of normal rats in the presence of a continuous [3H]oleate infusion and in isolated liver cells incubated in the presence of [3H]oleate. In liver perfusion experiments, 1 mM chlorpropamide enhanced the incorporation of tritium into triglycerides (but not other lipid classes) and increased both liver triglyceride content and triglyceride secretion. Using isolated cells similar effects could be demonstrated at 0.5 mM chlorpropamide. Chlorpropamide, tolbutamide, and carbutamide, all of which inhibited endogenous ketogenesis in isolated liver cells, also inhibited lysosomal triglyceride lipase activity in rat liver homogenates. The drugs were not inhibitory towards alkaline lipase activity. Demethylglycodiazin (2-benzolsulfonamid--5-(beta-hydroxyethoxy)-pyrimidin), which did not inhibit endogenous ketogenesis in isolated liver cells, did not affect lysosomal lipase activity. The lysosomotropic drug chloroquine was markedly antiketogenic when tested in liver cells. The reduction in endogenous ketogenesis, the enhanced accumulation of liver triglycerides, and the stimulation of hepatic triglyceride output by chlorpropamide are ascribed to an interference of the drug with hepatic triglyceride breakdown. The present results also suggest that the lysosomes play a significant role in hepatic lipolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMEYER H. U., BERNT E. ENZYMATISCHE BESTIMMUNG VON KETON-KOERPERN IM BLUT. Enzymol Biol Clin (Basel) 1965;19:65–76. [PubMed] [Google Scholar]
- BOSHELL B. R., ZAHND G. R., RENOLD A. E. An effect of tolbutamide on ketogenesis, in vivo and in vitro. Metabolism. 1960 Jan;9:21–29. [PubMed] [Google Scholar]
- BYERS S. O., FRIEDMAN M. Site of origin of plasma triglyceride. Am J Physiol. 1960 Mar;198:629–631. doi: 10.1152/ajplegacy.1960.198.3.629. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Colbeau A., Cuault F., Vignais P. M. Characterization and subcellular localization of lipase activities in rat liver cell. Comparison with phospholipase A. Biochimie. 1974;56(2):275–288. doi: 10.1016/s0300-9084(74)80388-3. [DOI] [PubMed] [Google Scholar]
- Debeer L. J., Mannaerts G., De Schepper P. J. Effects of octanoate and oleate on energy metabolism in the perfused rat liver. Eur J Biochem. 1974 Sep 16;47(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03730.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GREENBERG S. R., KLOTZ R. G., BEARDWOOD J. T., Jr Lipoprotein elevations of diabetics on tolbutamide as compared to lipoprotein levels during insulin therapy: preliminary observations. Am J Med Sci. 1961 Jun;241:718–726. doi: 10.1097/00000441-196106000-00004. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Guder W., Weiss L., Wieland O. Triglyceride breakdown in rat liver. The demonstration of three different lipases. Biochim Biophys Acta. 1969;187(2):173–185. doi: 10.1016/0005-2760(69)90025-3. [DOI] [PubMed] [Google Scholar]
- HEIMBERG M., WEINSTEIN I., KLAUSNER H., WATKINS M. L. Release and uptake of triglycerides by isolated perfused rat liver. Am J Physiol. 1962 Feb;202:353–358. doi: 10.1152/ajplegacy.1962.202.2.353. [DOI] [PubMed] [Google Scholar]
- Hasselblatt A. Die Hemmung der Ketogenese im Lebergewebe durch Tolbutamid und Glykodiazin in vitro. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;262(2):152–164. [PubMed] [Google Scholar]
- Hayase K., Tappel A. L. Specificity and other properties of lysosomal lipase of rat liver. J Biol Chem. 1970 Jan 10;245(1):169–175. [PubMed] [Google Scholar]
- Heimberg M., Van Harken D. R., Brown T. O. Hepatic lipid metabolism in experimental diabetes. II. Incorporation of [I-14C]palmitate into lipids of the liver and of the d less than 1.020 perfusate lipoproteins. Biochim Biophys Acta. 1967 Jun 6;137(3):435–445. [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood C. A., Warhurst D. C., Peters W., Baggaley V. C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972 Jan 7;235(5332):50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Kariya M., Kaplan A. Effects of acidic phospholipids, nucleotides, and heparin on the activity of lipase from rat liver lysosomes. J Lipid Res. 1973 Mar;14(2):243–249. [PubMed] [Google Scholar]
- Krauss R. M., Windmueller H. G., Levy R. I., Fredrickson D. S. Selective measurement of two different triglyceride lipase activities in rat postheparin plasma. J Lipid Res. 1973 May;14(3):286–295. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lie S. O., Schofield B. Inactivation of lysosomal function in normal cultured human fibroblasts by chloroquine. Biochem Pharmacol. 1973 Dec 1;22(23):3109–3114. doi: 10.1016/0006-2952(73)90197-4. [DOI] [PubMed] [Google Scholar]
- Mannaerts G., Debeer L., De Schepper P. J. Metabolic effects of hypoglycemic sulfonylureas. 3. Effect of chlorpropamide on energy metabolism and ketogenesis in the isolated perfused rat liver. Biochem Pharmacol. 1974 Jan 1;23(1):75–87. doi: 10.1016/0006-2952(74)90315-3. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- STEIN Y., SHAPIRO B. Assimilation and dissimilation of fatty acids by the rat liver. Am J Physiol. 1959 Jun;196(6):1238–1241. doi: 10.1152/ajplegacy.1959.196.6.1238. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Pfleger B. Utilization of exogenous free fatty acids for the production of very low density lipoprotein triglyceride by livers of carbohydrate-fed rats. J Lipid Res. 1971 Sep;12(5):614–621. [PubMed] [Google Scholar]
- Schotz M. C., Garfinkel A. S., Huebotter R. J., Stewart J. E. A rapid assay for lipoprotein lipase. J Lipid Res. 1970 Jan;11(1):68–69. [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Teng M. H., Kaplan A. Purification and properties of rat liver lysosomal lipase. J Biol Chem. 1974 Feb 25;249(4):1064–1070. [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]
- Waddell M., Fallon H. J. The effect of high-carbohydrate diets on liver triglyceride formation in the rat. J Clin Invest. 1973 Nov;52(11):2725–2731. doi: 10.1172/JCI107467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W. The isolation of hormone-sensitive rat hepatocytes by a modified enzymatic technique. Arch Biochem Biophys. 1974 Aug;163(2):600–608. doi: 10.1016/0003-9861(74)90519-0. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


