Abstract
Background
Persons with type 2 diabetes (T2D) are at risk for cognitive impairment and brain atrophy. The ACCORD Memory in Diabetes (MIND) Study investigated whether persons randomized to an intensive glycaemic therapeutic strategy targeting HbA1c to <6% had better cognitive function and a larger brain volume at 40 months than persons randomized to a standard strategy targeting HbA1c to 7%–7.9%.
Methods
ACCORD MIND was a double 2×2 factorial parallel group randomised trial conducted in 52 clinical sites in North America. Participants [age 55 – <80 years] with T2D, high HbA1c concentrations (>7.5%), and at high risk for cardiovascular events were randomised to treatment groups using a centralized web-based system. Clinic staff and participants were not blinded to treatment arm. The cognitive primary outcome, the Digit Symbol Substitution Test (DSST) score, was assessed at baseline, 20 and 40 months. Total brain volume (TBV), the primary brain structure outcome, was assessed with MRI at baseline and 40 months in a sub-set of 632 participants. All participants with follow-up data were included in the primary analyses. In February, 2008, increased mortality risk led to the termination of the intensive therapy and transition of those participants to standard glycaemic treatment.
Results
Randomised patients (n=2977; mean age 62.3 years) were consecutively enrolled; the final analysis included 1358 intensive and 1416 standard arm participants with a 20 or 40 month DSST score. Of the 614 with a baseline MRI, 230 intensive and 273 standard therapy participants were included in the analysis. There was no treatment difference in the DSST score. The intensive group had a greater TBV than the standard group (difference, 4.62; 95% CI 2.0 to7.3 cm3; p=0.0007).
Interpretation
Although significant differences in TBV favored the intensive therapy, cognitive outcomes were not different. Combined with the unfavorable effects on other ACCORD outcomes, MIND findings do not support using intensive therapy to reduce the adverse effects of diabetes on the brain in patients similar to MIND participants. (ClinicalTrials.gov number, NCT00182910).
Introduction
Studies (1) suggest that older persons with type 2 diabetes (T2D) have at least twice the likelihood of developing late-life cognitive impairment or dementia compared to those without T2D. The mechanisms underlying these cognitive disorders are increasingly thought to reflect a mixed pathology pattern with contributions from vascular, neurodegenerative, and neurovascular processes (2). Pathophysiological mechanisms that have been described include inflammation, oxidative stress, energy imbalance, protein misfolding, glucocorticoid-mediated effects, and differences in genetic susceptibilities (3, 4). Based on an extensive literature on the causes, management and prevention of diabetes, we took as a premise early intervention with therapeutic strategies that improve glyceamic control could mitigate the adverse effects of T2D on the brain. There are no clinical trials testing the effects of early intervention on brain outcomes in older persons with T2D. Targeting this risk group, the Memory in Diabetes (MIND) sub-study embedded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (5, 6) was designed to test the primary hypothesis that at 40 months, persons randomized to an intensive glycaemic therapeutic strategy targeting HbA1c to <6% have better cognitive function and a larger brain volume than persons randomised to a standard strategy targeting HbA1c to 7%–7.9%.
Methods
ACCORD , described elsewhere (6), is a randomised, multicenter, double two-by-two factorial parallel treatment trial that tested the effect on cardiovascular disease (CVD) events of therapeutic strategies to control blood glucose, blood pressure, and lipid levels. ACCORD targeted participants aged 45–79 yrs with T2D, high HbA1c concentrations (>7.5%), and at high risk for CVD events indicated by significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease. Key exclusion criteria included frequent or recent serious hypoglycemic events, unwillingness to monitor glucose at home or inject insulin, body-mass index >45, serum creatinine level >1.5 mg per deciliter (133 μmol per liter), or other serious illness (7).
All ACCORD participants (n=10,251) were randomly assigned to receive either the intensive glycaemic treatment strategy targeting HbA1c to <6% or the standard glycaemic treatment targeting HbA1c to 7.0– 7.9%. Additionally, using the double two-by-two factorial design, 4733 (46%) participants were randomly assigned to blood pressure (BP) lowering by receiving either intensive therapy (systolic BP target, <120 mm Hg) or standard therapy (SBP target, <140 mm Hg); 5518 (54%) participants were randomly assigned to receive either fenofibrate or placebo while maintaining good control of low2 density lipoprotein cholesterol with simvastatin (6).
ACCORD was implemented in 77 clinics spread across North America; each clinic was under one of seven clinical centre networks (CCN) and a central coordinating centre (CCC). The CCC computer generated unique randomisation sequences for every clinical site and electronically verified exclusion and inclusion criteria for every individual before assigning a treatment group. Clinic staff implemented the randomisation via secure access to the ACCORD trial website. Glycaemia trial treatment assignment was open label, and both clinic staff and patients were aware of the assigned glycaemic goal. Study investigators were masked to results of all ACCORD interim analyses.
The ACCORD therapeutic intervention achieved the target HbA1c using a variety of strategies decided by the attending physician and tailored to the individual. All participants received diabetes education, glucose-monitoring equipment, and antidiabetic medications. Participants in the intensive glycaemic group were started on ≥2 classes of agents. Doses were intensified or a new medication class was added monthly if HbA1c levels were ≥6%, or if >50% of premeal or postmeal capillary glucose readings were >5.6 mmol/L (100 mg/dL). Standard glycaemic therapy was intensified whenever HbA1c was ≥8%, or >50% of capillary glucose readings were >7.8 mmol/L (140 mg/dL). Antihyperglycemic drugs that promoted hypoglycemia (i.e., insulin or insulin secretagogues) were reduced if HbA1c was persistently lowered to <7%. All drug combinations from a standard formulary were permitted; specific drugs were reduced only for side effects or contraindications (8).
The intensive intervention was stopped in February, 2008 when an increased risk (HR, 1.22) for mortality was reported; participants in that arm were transitioned to the standard glycaemic treatment (7). MIND evaluations continued according to the original protocol. Here we report the glycaemia result since this was the main intervention for which MIND was powered. Results for the other interventions will be reported elsewhere.
The National Heart Lung and Blood Institute (NHLBI) sponsored ACCORD and a NHLBI review panel and the institutional review board (IRB) or ethics committee at each participating centre approved the protocol. The National Institute on Aging in collaboration with NHLBI sponsored the MIND trial, which was approved by the IRB of all participating institutions (Appendix 1). Participants signed a separate informed consent for MIND.
ACCORD MIND
The MIND study design has been described (5). All randomised ACCORD participants were eligible for MIND if they were recruited between August 21, 2003 (34 months after the start of ACCORD) and December 16, 2005 when the target sample size was reached. From this pool, we excluded participants aged <55 years and those in the Veteran’s Administration CCN because participants were projected to be mainly male and we wanted to retain the overall sex balance reflected in the other CCNs. This resulted in MIND eligibility of 54% (n=5575) of the whole ACCORD sample. These participants were drawn from 52 North American clinics in six of the seven CCN (see Appendix 1). Within MIND, a sub12 sample from four CCNs (28 clinics) participated in the MRI sub-study. A cognitive test battery was administered at baseline, 20 months and 40 months post-randomisation. Brain MRIs were acquired at baseline and at 40 months.
Cognitive Function
Eligible randomised participants were enrolled into MIND consecutively until the recruitment goal was reached. The cognitive battery tested for verbal memory, processing speed, and executive function, which are typically impaired in persons with T2D (9). Specific test selection, described in more detail elsewhere (5), took into consideration the context of testing in multiple clinic sites by trained and standardized lay clinic staff; clinic time and patient burden; as well as having been previously used in studies of cognition and diabetes (10). The primary cognitive outcome was the number of correctly completed cells on the 40-month Digit Symbol Substitution Test (DSST), an omnibus test of psychomotor speed that also requires reasoning and working memory (11). This test has a normal distribution in the age group of MIND participants, has been shown to change over time, is associated with diabetes and other cardio-vascular outcomes, and may be less sensitive to educational level (12). Secondary cognitive outcomes were memory measured with the Rey Auditory Verbal Learning Test (RAVLT) and executive function measured with the Stroop test (5). The widely-used Mini Mental State Examination of global cognitive function was administered to allow comparisons with other studies.
Quality control (QC) by the MIND Coordinating Centre, described elsewhere (5, 13) included tester (re)certification, review of recorded test sessions, a tester helpdesk at the Coordinating Centre, and continual review of data entry and test score distributions for unusual trends.
MRI
Based on the evidence that diabetes can lead to mixed pathology of vascular and neurodegenerative changes (14, 15), evidence of change over time (16), and its relationship to cognitive function and decline, total brain volume (TBV, cm3) was chosen as the primary MRI endpoint. Rates of whole brain atrophy have been shown to be sensitive and powerful markers of disease progression (17, 18) and to differ between persons with and without diabetes (19, 20); smaller values predict future cognitive disorders (21). The secondary MRI outcome was abnormal white matter (AWM) tissue volume, which reflects diffuse and focal ischemic, demyelinating, and inflammatory processes leading to small vessel disease, and is associated with diabetes and impaired cognition (20, 22).
Eligible participants were recruited continuously from the cognitive study participants. Initially only participants randomised to the glycaemic and blood pressure trials were targeted, but halfway through the study, recruitment was extended to lipid trial participants to meet sample size goals. Participants with standard MRI exclusions were excluded (23). To enhance retention, recruitment focused on participants living within two hours of the MR scanner.
The standardized MRI scan protocol (5), acquired on all subjects, was run on 1.5T scanners and included a 3-D fast spoiled gradient-echo (SPGR) T1-weighted sequence (21; 30; 8:TR; FA; TE), 2-D axial fast spin23 echo (FSE) fluid attenuated inversion recovery (FLAIR) (8,000; 2,000; 100; TR; TI; TE), and proton24 density/T2 (3,200; 27, 120; TR; TE1,2) weighted sequences. Voxel size for the 3D T1 sequence is 1.5 × 0.9 × 0.9 mm and 3.0 × 0.9 × 0.9 mm for the 2D sequences. In general, the 3D T1 sequence is used to study brain morphology, including volume, and the FSE sequences are used to study pathology.
An operator ran the standardized MR sequences that were programmed into the scanner and did not change during the study. MRI quality control (QC) followed the American College of Radiology’s (ACR) MRI QC Program, (http://www.acr.org/accreditation/mri.aspx). Monthly digital images acquired at each Field Center were sent to the MRI QC center for in-house review. According to ACR phantom analyses, MRI scanner performance was stable across MRI sites and over the duration of the study.
The image analysis was performed using methodology previously described (24) (25), based on an automated multi-spectral computer algorithm that classifies all supratentorial brain tissue into 92 volumetric (cc3) anatomical regions of interest (ROI) characterized as cerebrospinal fluid , or gray or white matter. Gray and white matter were further characterized as normal and abnormal. Abnormal white matter (AWM) reflected both diffuse small vessel disease, and the hyperintensities that surround focal lesions. Gray and white matter ROI’s were summed to estimate total brain volume (TBV); TBV and CSF were summed to estimate intracranial volume (ICV), a measure of head size. Each participant’s processed scan was reviewed by a trained individual who removed any scans verified to have failed to reach a stable solution (n=18).
ICV, an integrated metric of the stability of both MRI operator/scanning and image analysis, did not significantly change between baseline and follow-up examinations (Baseline mean ICV, 1132.34 cm3; follow-up mean ICV, 1132.32 cm3; p=0.4651 by paired t-test).
Sample size
The sample size of 1400 participants per glycaemia group was estimated to detect at 40 months an 18% difference between glycaemia groups (1 point) with approximately 90% power, assuming a two-sided 0.05 type 1 error level, 15% dropout and a 40-month DSST SD of 7.5 points, adjusted for baseline DSST; a total of 2977 were recruited.
The MIND MRI sample size of 320 participants per glycaemia group was estimated to detect a 20% 40-month TBV difference (3.3 cm3) between groups, with approximately 90% power, assuming a two-sided 0.05 type 1 error level, 15% dropout, and a TBV SD of 12.1, adjusted for baseline TVB (16); 632 participants were recruited.
Statistical Analyses
All statistical analyses were conducted with S-Plus 8.0 (Insightful Corp., Seattle, WA) or SAS 9.2 (SAS Institute, Cary, NC).
The cognitive function hypotheses were tested with a mixed effects model that incorporated information from both the 20 and 40 month outcome measures (26). This model assumes the probability of missing outcomes depends only on prior observed outcomes or on factors in the model. The basic model included a term for the glycaemia intervention, a visit effect, and an interaction term between the two. In a randomised trial the baseline covariates are independent of the random assignment (27), so we could improve the efficiency of the analysis by including in the model the baseline cognitive score and the factors used to stratify randomization: second trial assignment (BP or Lipid); randomised group allocation within the BP and lipid trials respectively; CCN ; and history of CVD.
The MRI hypotheses were tested using an analysis of covariance model that included ICV and factors used to stratify randomization, as described above. The highly skewed baseline and 40 month AWM data were log transformed; we present the back-transformed estimates of treatment differences, which is the ratio of the treatment-specific geometric means (28).
Robustness of the MRI results to missing 40-month data (including those due to death) was investigated in three multiple imputation regression models that used baseline MRI information for imputation. In one model imputation was based on data pooled across treatment groups; a second based imputation on data from each treatment arm separately; a third investigated how much change in TBV would have been needed in the intensive glycaemia participants missing 40 month data for the treatment comparison to no longer be significant. Following the finding that participants in the intensive arm gained more weight than those in the standard arm (7), post-hoc exploratory analyses were performed for treatment differences in edematous conditions [pre-tibial edema, worsened ankle swelling, CHF, pulmonary edema, new or worsened shortness of breath, or nocturia], or whether within treatment groups, weight gain was associated with TBV and AWM.
Pre-specified sub-group analyses were conducted for gender, history of CVD, treatment arm in the lipid/BP trials, and CCN. Post-hoc exploratory sub-group analyses included baseline: age [< 60, 60–69, 70+ yrs] (29); duration of diabetes [<5, 6–10, 11–15,16+ yrs] (13, 30) and DSST [<47, 47–59, 60+] (31).
All hypotheses were tested at the two-sided 0.05 level.
Role of funding source: Staff from the National Heart Lung and Blood Institute (ACCORD sponsor) served on the Executive and Steering Committees that made decision on study design, methodology and data collection. The MIND sponsor (National Institute on Aging) had no role in the study design, collection, analysis and interpretation of the data, in writing the report, or the decision to submit the paper for publication. No author, except Dr Gerstein, has any conflicts of interest.
Results
Participants in the total MIND sample
ACCORD-MIND participants were similar to the overall eligible ACCORD sample (eTable 1) and treatment groups were similar to each other (Table 1). The substantial separation in median HbA1C between the intensive (6.6%) and standard (7.5%) groups was similar to that achieved in the main trial. On February 8, 2008 when the intensive glycaemic intervention was stopped, participants in that arm were transitioned to the standard glycaemic treatment (7). At the time of the transition, cognitive study intensive group participants were on treatment for a median of 39 months (percentiles: 25th = 34; 75th = 40); and MRI participants were on treatment for 35 months (31, 40). Mortality in the intensive vs. standard group (HR=1.27; 95% CI 0.83 to 1.93; 47 deaths in the intensive arm and 39 in the standard arm) MIND participants was consistent with that observed overall in ACCORD.
Table 1.
Baseline Characteristics of the ACCORD-MIND Cohort
| Glycemia Intervention | ||
|---|---|---|
| Intensive | Standard | |
| Number (%) | 1469 (49.3) | 1508 (50.7) |
| Age – mean (sd) years | 62.3 (5.7) | 62.7 (5.9) |
| Sex : N (%) female | 697 (47.5) | 691 (45.8) |
| Education, N (%) | ||
| Less than HS graduate | 208 (14.2) | 184 (12.2) |
| HS graduate/GED | 374 (25.5) | 395 (26.2) |
| Some college/tech | 512 (34.9) | 515 (34.2) |
| College graduate or more | 375 (25.5) | 414 (27.5) |
| Network, N (%) | ||
| Canada | 153 (10.4) | 147 (9.8) |
| Western | 300 (20.4) | 310 (20.6) |
| Minn/Iowa | 329 (22.4) | 345 (22.9) |
| Ohio/Mi | 172 (11.7) | 187 (12.4) |
| Northeast | 194 (13.2) | 180 (11.9) |
| Southeast | 321 (21.9) | 339 (22.5) |
| Race/ethnicity, N (%) | ||
| White | 1020 (69.4) | 1054 (69.9) |
| Black | 242 (16.5) | 242 (16.1) |
| Hispanic | 105 (7.2) | 107 (7.1) |
| Other | 102 (6.9) | 105 (6.9) |
| Smoking Status, N (%) | ||
| Current | 172 (11.7) | 180 (11.9) |
| Former | 641 (43.7) | 654 (43.4) |
| Never | 654 (44.6) | 673 (44.7) |
| Systolic BP – mean (sd) mm Hg | 135.3 (17.3) | 135.7 (18.2) |
| Diastolic BP – mean (sd) mm Hg | 75.1 (10.5) | 74.5 (10.8) |
| Duration diabetes‡ | 9 (5–14) | 9 (5–15) |
| HbA1c (%)– mean (sd) | 8.3 (1.0) | 8.3 (1.1) |
| Total Cholesterol (mmol/L) | 4.73 (1.05) | 4.75 (1.12) |
| Low-density lipoprotein (mmol/L) | 2.68 (0.86) | 2.67 (0.88) |
| High-density lipoprotein (mmol/L) | ||
| Women | 1.22 (0.31) | 1.22 (0.31) |
| Men | 0.99 (0.25) | 1.00 (0.23) |
| BMI – mean (sd) | 33.0 (5.4) | 32.9 (5.3) |
| History of CVD, N (%) | 427 (29.1) | 442 (29.3) |
| Depression N (% PHQ > 10)†, N (%) | 215 (14.6) | 226 (15.0) |
| Baseline DSST† | 52.5 (15.7) | 52.6 (16.1) |
| Baseline RAVLT† | 7.6 (2.6) | 7.5 (2.5) |
| Baseline Stroop† | 32.4 (17.4) | 31.6 (15.9) |
| Baseline Mini Mental†‡ | 28 (26–29) | 28 (26–29) |
DSST: number of correct cells (possible range 0 – 90); RAVLT: total number of words recalled (possible range 0 to 15); Stroop: Interference score; MMSE: possible range 0 – 30; PHQ: possible range 0 – 27
median (25th and 75th percentile)
Cognitive function outcomes
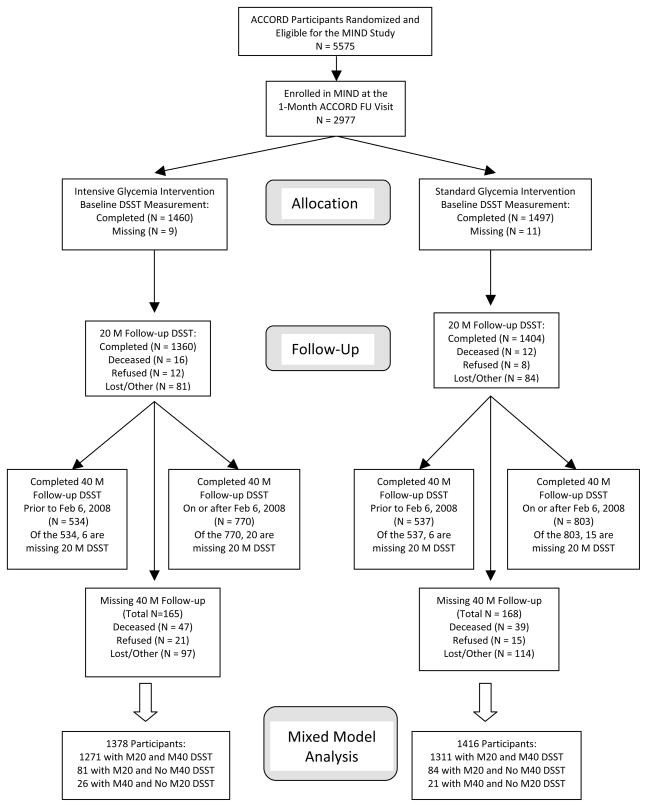
A baseline DSST was obtained on 2,957 (99%) MIND participants (Figure 1); 94% (1378 intensive and 1416 standard) had at least one (20- or 40-month) follow-up and were included in the final analysis. Completion rates for the other tests were similar to the DSST. Those with missing follow-up data were older, had a higher systolic BP and a lower baseline DSST but were otherwise similar to those with complete data.
Figure 1.
ACCORD-MIND Cohort Participation in the Primary Cognitive Outcome
DSST scores significantly declined in both intervention arms (Table 2a). At 20 months the between-group difference in DSST scores approached statistical significance but at 40 months the difference was attenuated and not significant (Table 2a). There were no consistent sub-group differences by intervention arm (eFigure 1).
Table 2.
Cognitive and MRI Primary and Secondary Outcomes by Glycaemia arm
| Table 2a | ||||
|---|---|---|---|---|
| Endpoint | Time Point | Glycemia Intervention
|
Difference in Means† | |
| Intensive* | Standard* | |||
| DSST | Baseline‡ | 52.55 | 52.55 | |
| 20 – Months | 51.51 (51.09 to 51.93) | 50.98 (50.57 to 51.39) | 0.53 (−0.06 to 1.12) p = 0.0756 | |
| 40 – Months | 50.93 (50.50 to 51.35) | 50.61 (50.19 to 51.03) | 0.32 (−0.28 to 0.91) p = 0.2997§ | |
| 40 Month Change | −1.62 (−2.05 to −1.20) | −1.94 (−2.36 to −1.52) | ||
| RAVLT | Baseline‡ | 7.51 | 7.51 | |
| 20 – Months | 7.87 ( 7.77 to 7.96) | 7.85 ( 7.76 to 7.94) | 0.02 (−0.11 to 0.15) p = 0.7897 | |
| 40 – Months | 7.98 ( 7.88 to 8.08) | 7.99 ( 7.90 to 8.08) | −0.01 (−0.14 to 0.12) p = 0.8929 | |
| 40 Month Change | 0.47 ( 0.37 to 0.57) | 0.48 ( 0.39 to 0.57) | ||
| Stroop | Baseline‡ | 32.0 | 32.0 | |
| 20 – Months | 30.87 (30.16 to 31.57) | 31.46 (30.77 to 32.16) | −0.60 (−1.59 to 0.40) p = 0.2375 | |
| 40 – Months | 31.45 (30.73 to 32.17) | 32.06 (31.34 to 32.77) | −0.61 (−1.62 to 0.40) p = 0.2383 | |
| 40 Month Change | −0.55 (−1.27 to 0.17) | 0.06 (−0.66 to 0.77) | ||
| MMSE | Baseline‡ | 27.39 | 27.39 | |
| 20 – Months | 27.26 (27.14 to 27.38) | 27.27 (27.15 to 27.39) | −0.01 (−0.18 to 0.16) p = 0.9268 | |
| 40 – Months | 27.05 (26.93 to 27.17) | 27.06 (26.93 to 27.18) | −0.01 (−0.18 to 0.16) p = 0.9328 | |
| 40 Month Change | −0.34 (−0.46 to −0.22) | −0.33 (−0.46 to −0.21) | ||
| MRI-TBV | Table 2b | |||
| Baseline‡ | 927.5 | 927.5 | ||
| 40 – Months | 914.4 (912.5 to 916.4) | 909.8 (908.0 to 911.6) MRI-TBV | 4.6 (2.0 to 7.3) p < 0.0007§ | |
| 40 Month Change | −13.0 (−15.0 to −11.1) | −17.7 (−19.5 to −15.9) | ||
Least Squares Mean (95% CI). For DSST, RAVLT, and MMSE a negative change value represents a decline in cognitive score. For the Stroop test, a positive change value represents a worsening score. For TBV, a negative change value represents a decline in volume.
Difference calculated as Intensive minus Standard arm means.
Baseline mean is the overall mean for both groups combined as measured pre-randomization. This value is used to obtain the least squares means estimates at follow-up. Models are adjusted for baseline cognitive score and the factors used to stratify randomization: second trial assignment (BP or Lipid); randomised group allocation within the BP and lipid trials respectively; CCN ; and history of CVD . Observed baseline means within each group are presented in Table 1.
Test of pre-specified co-primary outcome.
Over the follow-up, there was a small increase in mean RAVLT scores within both glycaemic groups but no significant difference between groups (Table 2a). Performance on the Stroop interference test improved slightly in the Intensive group and declined slightly in the standard group, but there was no difference between treatments (Table 2a). There were generally no consistent sub-group differences by intervention arm for either of these cognitive tests (eFigures 2–3).
MRI outcomes
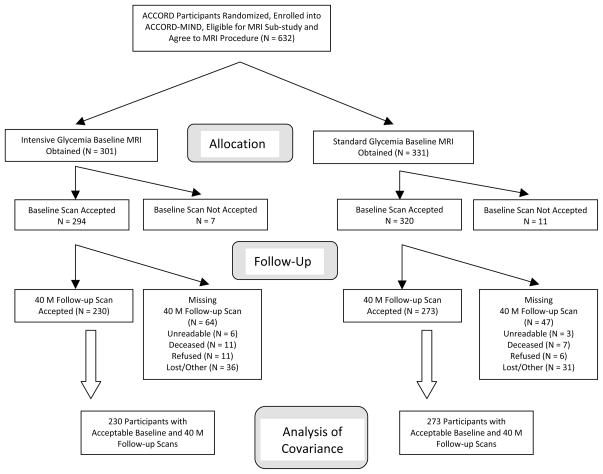
Of the 632 participants recruited into the MRI sub-study, 614 (97%) MIND participants (294 in the intensive, and 320 in the standard glycaemia groups; Figure 2) had a successful baseline MRI and were similar for baseline characteristics to all other MIND subjects (eTable 2) and between treatment groups (Table 3).
Figure 2.
ACCORD MIND Cohort Participation in the Primary MRI Outcome
Table 3.
Baseline Characteristics of the ACCORD MIND MRI Cohort
| Glycemia Intervention | ||
|---|---|---|
| Intensive | Standard | |
| Number (%) | 294 (47.9) | 320 (52.1) |
| Age – mean (sd) years | 62.1 (5.7) | 62.7 (5.8) |
| Sex : N (%) female | 130 (44.2) | 143 (44.7) |
| Education, N (%) | ||
| Less than HS graduate | 32 (10.9) | 30 (9.4) |
| HS graduate/GED | 69 (23.5) | 79 (24.7) |
| Some college/tech | 103 (35.0) | 107 (33.4) |
| College graduate or more | 90 (30.6) | 104 (32.5) |
| Network, N (%) | ||
| Minn/Iowa | 129 (43.9) | 145 (45.3) |
| Ohio/Mi | 46 (15.7) | 53 (16.6) |
| Northeast | 48 (16.3) | 38 (11.9) |
| Southeast | 71 (24.2) | 84 (26.3) |
| Race/ethnicity, N (%) | ||
| White | 192 (65.3) | 228 (71.3) |
| Black | 53 (18.0) | 50 (15.6) |
| Hispanic | 22 (7.5) | 17 (5.3) |
| Other | 27 (9.2) | 25 (7.8) |
| Smoking Status, N (%) | ||
| Current | 38 (13.0) | 42 (13.1) |
| Former | 123 (42.0) | 135 (42.2) |
| Never | 132 (45.1) | 143 (44.7) |
| Systolic BP – mean (sd) mm Hg | 133.5 (16.3) | 136.0 (19.2) |
| Diastolic BP – mean (sd) mm Hg | 74.5 (9.8) | 74.5 (10.6) |
| Duration diabetes‡ | 8 (5–13) | 8 (5–13) |
| HbA1c – mean (sd) | 8.2 (1.0) | 8.1 (1.0) |
| Total Cholesterol (mmol/L) | 4.71 (1.00) | 4.75 (1.16) |
| Low-density lipoprotein (mmol/L) | 2.62 (0.82) | 2.65 (0.90) |
| High-density lipoprotein (mmol/L) | ||
| Women | 1.28 (0.35) | 1.24 (0.32) |
| Men | 1.01 (0.28) | 1.04 (0.24) |
| BMI – mean (sd) | 33.1 (5.1) | 32.2 (5.0) |
| History of CVD, N (%) | 82 (27.9) | 78 (24.4) |
| Total brain volume (cm3) | 928.3 (101.2) | 926.8 (91.5) |
| Depression [PHQ % > 10]†, N (%) | 45 (15.3) | 48 (15.0) |
| DSST† | 52.5 (15.9) | 54.2 (16.2) |
| RAVLT total† | 7.4 (2.5) | 7.5 (2.5) |
| Stroop† | 32.1 (17.1) | 29.9 (13.8) |
| MMSE†‡ | 28 (26 – 29) | 28 (27 – 29) |
DSST: number of correct cells (possible range 0 – 90); RAVLT: total number of words recalled (possible range 0 to 15); Stroop: Interference score; MMSE: possible range 0 – 30; PHQ: possible range 0 – 27
median (25th and 75th percentile)
Of the 614 MRI participants, a higher proportion (p=0.0273) of standard compared to intensive therapy participants had a successfully processed repeat scan (273 (85.3%) vs. 230 (78.2%) respectively). Reasons for missing scans (eTable 3) were similarly distributed across treatment arms. More older compared to younger participants were missing a follow-up scan (15.1% (n=35) of the 232 <60 vs. 19.8% (n=76) of the 382 ≥60 year olds).
At 40 months, the intensive therapy group had significantly greater TBV compared to the standard group (Table 2b). Although TBV declined in both groups, the intensive glycaemic group TBV declined less, by 13.0 cm3 (0.41%/yr) compared to 17.7 cm3 (0.57%/yr) in the standard group TBV; Imputation based sensitivity analyses showed similar results; the intensive participants missing a 40 month MRI would have had to have experienced, on average, a > 22.0 cm3 decline (73% increase over the change In those with observed data) for the results to become non-significant. The effect on TBV of the interventions did not differ by sub-group (prior CVD: p=0.1508; gender: p=0.6336; CCN: p=0.6509; diabetes duration: p=0.7167; age: p=0.4824; DSST: p=0.4650).
At 40 months, there was significantly more AWM in the intensive (geometric mean 1.89 cm3 95% CI 1.78 to 2.00) compared to standard (1.71 95% CI 1.62 to 1.80) therapy group (ratio of geometric means = 1.10 cm3, 95% CI 1.02 to 1.19; p<0.0156). However, this effect appeared to be restricted to participants < 60 years old (interaction between the glycaemia intervention and baseline age (p=0.0045; ratio of intensive to standard geometric means <60 years old (n= 197) = 1.30 (95% CI 1.15 to 1.48), 60–69 years (n= 245) = 0.98 (95% CI 0.87 to 1.09), and 70+ years (n= 61) = 1.07 (95% CI 0.84 to 1.35). There were no other treatment differences across baseline subgroups (prior CVD: p=0.35; gender: p=0.82; CCN: p=0.3401; diabetes duration: p=0.7496; DSST: p=0.8073).There was no evidence that measures of peripheral edema or weight gain could explain the between treatment arm differences in TBV or AWM.
Discussion
ACCORD MIND is the first randomised trial in older persons with T2D to test the effect of intensive compared to standard glycaemic therapeutic strategies on multiple cognitive domains and on structural changes in the brain. Overall, there is no evidence that in this patient group with long-standing T2D, high CVD risk and mean age 62 years, an intensive glycaemic therapeutic strategy provides benefit to cognitive function. There was a significant but small difference in TBV favoring the intensive strategy. This difference would not support using intensive therapy as a means to reduce brain atrophy when taking into account the effects this intervention had in the main ACCORD trial, including increased mortality, no overall benefit on CVD events, an increase in hypoglycaemic events and weight gain (7).
The MIND 30% sub-sample of ACCORD participants, the separation in HbA1c levels, and differences in mortality rates between the treatment strategy groups were similar to the main trial. There was reasonable balance of baseline characteristics between treatment groups. Adherence and retention to the cognitive assessment protocol was high, minimizing the likelihood of bias. The cognitive battery was successfully administered in a standardized manner in a large number of geographically and demographically diverse clinics; there were fewer missing 40-month DSST assessments than expected (11.1% (n=333) actual vs. 15% expected), and these were distributed similarly across the treatment arms (11.2% (n=165) intensive vs. 11.1% (n=168) standard strategies). The overall conclusions did not change with different assumptions about the missing 40-month scans.
Several factors may have attenuated treatment differences in cognitive scores. Not all participants completed 40 months on intensive therapy, but most had at least 34 months of therapy. Methodologic factors, such as practice effects, may contribute but these effects should be similar in both treatment arms. Possibly the tests did not measure the appropriate functions but those functions have been repeatedly shown to be affected in T2D (9) and the tests are appropriate for a large-scale heterogeneous study population. For the deaths to have influenced our conclusion in favor of intensive therapy, the 47 decedents in the intensive group would have had to have considerably higher follow-up cognitive scores than the 39 decedents in the standard group. We think this is unlikely because it assumes the 8 excess deaths would have had to specifically occur in persons whose MIND outcomes would have benefited from intensive therapy.
There are several other explanations to consider. High patient motivation and the optimal diabetes care provided to all participants may have brought glucose into sufficient control to have mitigated some cerebral pathology caused by T2D (3). Indeed optimal treatment has been raised as a reason for the null effect on cognition in the DCCT/EDIC trial (32). Age may also be a factor insofar as treatment differences would be more apparent had they been given during a period when participants were experiencing more rapid decline in cognition (33). It has been suggested that up to age 70 years, there is little measurable cognitive decline in persons with T2D, although after that the rates of decline begin to diverge between those who remain cognitively stable and those who will develop MCI or AD. Possibly intensive treatment was not efficacious given the relatively advanced disease and the elevated risk for CVD.
The annualized decline in TBV (3.9 cm3) in the intensive group is 26% less than the annualized TBV change in the standard treatment group (5.31 cm3). From another perspective, a study of persons mean age 76 years, found TBV of cognitively stable persons declined 0.4%/yr compared to 0.8%/yr in those who converted to Mild Cognitive Impairment or dementia (34). This is compared to an annual decline of 0.42% in the intensive and 0.57% in the standard group. The increase in AWM volume in participants < 60 years in the intensive group requires further study. We found no evidence that major factors, edema or weight gain, influenced the results, although there may have been some other unknown or unmeasured side effect that may result in TBV treatment differences.
Taking cognitive and MRI findings together it is reasonable to hypothesize that in this age group structural brain changes occur before cognitive change and that over time cognitive differences between treatment arms will emerge. With additional on-going follow-up of the cohort, we will be able to determine whether, above overall good glyceamic control, the different treatment strategies resulted in different rates of cognitive change.
At this time, there is scant evidence to quantify the clinical impact of the observed treatment differences. It is reasonable to suggest that a larger decline in brain capacity will lead to earlier loss of function and possibly dementia – the MIND participants at an average of 62 years old are already experiencing an annual decline of TBV in the range reported for persons 15 years older (34), when the incidence of dementia increases logarithmically. In addition, there are few data quantifying the trajectory of brain changes in persons with T2D who are similar in age to MIND participants, the functional effects of accumulating small decrements in brain structure and function, and the determinants of who, in a general population, will go on to dementia. Most data on persons with diabetes describe trajectories in (younger) type 1 diabetes (35), or in cohorts that are at least 10 years older(1). However, the age of MIND participants is the critical period when disease processes in the brain begin to accelerate eventually leading to the observed two times risk of dementia in persons with T2D compared to normotensives –and it is this transition phase where there are clearly gaps in our knowledge that need to be filled if we are to design effective prevention strategies.
Cognitive function influences the ability of patients to follow complex disease management protocols, and impaired cognition predicts CVD and severe hypoglycaemic events (36). Early prevention strategies reducing the risk for cognitive impairment are needed as the longevity of this diabetic group increases along with the number reaching an age when cognitive disorders become clinically apparent. Optimal therapeutic strategies for brain health in older persons with T2D are needed and should be evaluated in the context of the risks and benefits on other sequelae of T2D.
Supplementary Material
Acknowledgments
Funding
ACCORD MIND was funded through an intra-agency agreement between NIA and NHLBI (AG-0002) and the NIA Intramural Research Program. ACCORD was funded by NHLBI (N01-HC-95178; N01-HC-95179; N01-HC-95180; N01-HC-95181; N01-HC-95182; N01-HC-95183; N01-HC-95184). The funding agencies had no role in the results reported here. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Schering-Plough.
Footnotes
Author Contributions
Study design – Lenore J. Launer, Jeff Williamson, R. Nick Bryan, Mike Miller
Data collection – Ronald M. Lazar, Hertzel C. Gerstein, Anne M. Murray, Mark D. Sullivan, Karen R. Horowitz, Jingzhong Ding, Chip Truwit, Jeffrey L. Sunshine, Joseph Maldjian, Joy Hirsch
Quality control and data analysis – Laura Coker, Mike Miller, Laura C. Lovato, James Lovato, Christos Davatzikos
Data interpretation and writing – LJL was lead author with MM, JW and RNB, All authors contributed equally to the interpretation of the data.
Conflicts of Interest
Dr. Hertzel C. Gerstein reported receiving consulting fees from Sanofi-Aventis, GlaxoSmithKline, Eli Lilly, Novo Nordisk, AstraZeneca, Bristol-Myers Squibb, Roche, Merck, Bayer, and Janssen-Ortho, institutional grant support to McMaster University from Sanofi-Aventis, GlaxoSmithKline, Novo Nordisk, Merck, Pronova, Roche, Eli Lilly, and Boehringer Ingelheim, and lecture fees from Sanofi-Aventis, GlaxoSmithKline, Eli Lilly and Novo Nordisk.
Dr. Launer had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006 Jan;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 2.Strachan MW R D Lawrence Lecture. The brain as a target organ in Type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2010 Feb;28(2):141–7. doi: 10.1111/j.1464-5491.2010.03199.x. [DOI] [PubMed] [Google Scholar]
- 3.Klein JP, Waxman SG. The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol. 2003 Sep;2(9):548–54. doi: 10.1016/s1474-4422(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 4.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006 Jan;100(1):328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 5.Williamson JD, Miller ME, Bryan RN, Lazar RM, Coker LH, Johnson J, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007 Jun 18;99(12A):112i–22i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007 Jun 18;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007 Jun 18;99(12A):34i–43i. doi: 10.1016/j.amjcard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005 Dec;48(12):2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 10.Coker LH, Shumaker SA. Type 2 diabetes mellitus and cognition: an understudied issue in women's health. J Psychosom Res. 2003 Feb;54(2):129–39. doi: 10.1016/s0022-3999(02)00523-8. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D, editor. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1988. [Google Scholar]
- 12.Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and digit symbol substitution performance: a meta-analysis. Psychol Aging. 2004 Mar;19(1):211–4. doi: 10.1037/0882-7974.19.1.211. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009 Feb;32(2):221–6. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008 Nov 15;43(3):470–7. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet. 2004 Jan 31;363(9406):392–4. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 16.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003 Apr 15;23(8):3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. Feb;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, et al. Volumetric MRI and cognitive measures in Alzheimer disease : comparison of markers of progression. J Neurol. 2008 Apr;255(4):567–74. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- 19.Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, Kjartansson O, et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009 Sep;32(9):1608–13. doi: 10.2337/dc08-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006 Nov;29(11):2539–48. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. Nov;133(11):3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawyer-Glover AM, Shellock FG. Pre-MRI procedure screening: recommendations and safety considerations for biomedical implants and devices. J Magn Reson Imaging. 2000 Jul;12(1):92–106. doi: 10.1002/1522-2586(200007)12:1<92::aid-jmri11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998 Sep-Oct;22(5):827–37. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008 Mar;15(3):300–13. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GM . Missing Data in Clinical Studies. Chichester: John Wiley and Sons Ltd; Chichester, England: 2007. [Google Scholar]
- 27.Fitzmaurice G. A conundrum in the analysis of change. Nutrition. 2001 Apr;17(4):360–1. doi: 10.1016/s0899-9007(00)00593-1. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. The use of transformation when comparing two means. BMJ. 1996 May 4;312(7039):1153. doi: 10.1136/bmj.312.7039.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000 Oct;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 30.Saczynski JS, Jonsdottir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility--Reykjavik study. Am J Epidemiol. 2008 Nov 15;168(10):1132–9. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, et al. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE) Am J Hypertens. 2005 Aug;18(8):1052–9. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007 May 3;356(18):1842–52. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008 Feb;7(2):184–90. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004 Feb 24;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan CM. Diabetes, aging, and cognitive decline. Neurobiol Aging. 2005 Dec;26(Suppl 1):21–5. doi: 10.1016/j.neurobiolaging.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.de Galan BE, Zoungas S, Chalmers J, Anderson C, Dufouil C, Pillai A, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009 Nov;52(11):2328–36. doi: 10.1007/s00125-009-1484-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.