Abstract
Insulin, as well as other ligands which increase intracellular guanosine 3',5'-cyclic monophosphate (cGMP), augments thymic-derived (T)- lymphocyte effector activity as revealed by alloimmune lymphocyte-mediated cytotoxicity. The observation that insulin binds only to monocytes among circulating nonimmune human mononuclear cells fosterd reexamination of the mechanism by which insulin augments T-lymphocyte function. This report concerns a test of the hypothesis that the T cell is directly affected by insulin and that an insulin receptor emerges upon T lymphocytes consequent to immune activation. Spleens were removed from rats skin grafted across a major histocompatibility barrier. Lymphocytes were harvested from Ficoll-Hypaque density gradients and subsequently enriched for T cells by passage over one or two nylon wool columns. This population was composed of more than 98% T cells as assessed by surface marker techniques (Ig staining, erythrocyte antibody, and erythrocyte antibody complement rosetting, anti-T staining). There was no loss of augmentation of lymphocyte-mediated cytotoxicity induced by insulin, carbamycholine, and 8-bromo-cGMP in the purified cells when compared to unfractionated cells 7 days after transplantation. 125I-insulin bound saturably to the allostimulated T-enriched lymphocytes with maximum binding at 12.8 +/- 0.2 pg and a dissociation constant at equilibrium of 1.3 nM. In contrast, insulin receptors were not present on nonimmune T-enriched cells or on T cells from animals that received syngeneic grafts. The affinity of the lymphocyte insulin receptor was similar to that of more conventional insulin-sensitive tissues e.g., liver, adipocyte. After 89% of T cells from spleens on day 7 were lysed with anti-thy 1.1 antibody and complement, the ability to measure specific insulin binding was lost. These data confirm a physiologic role for insulin in T-lymphocyte effector function and describe the emergence of insulin receptors concomitant with cell sensitivity to ligand. Such receptors may play a role in hormonal modulation of the immune response.
Full text
PDF


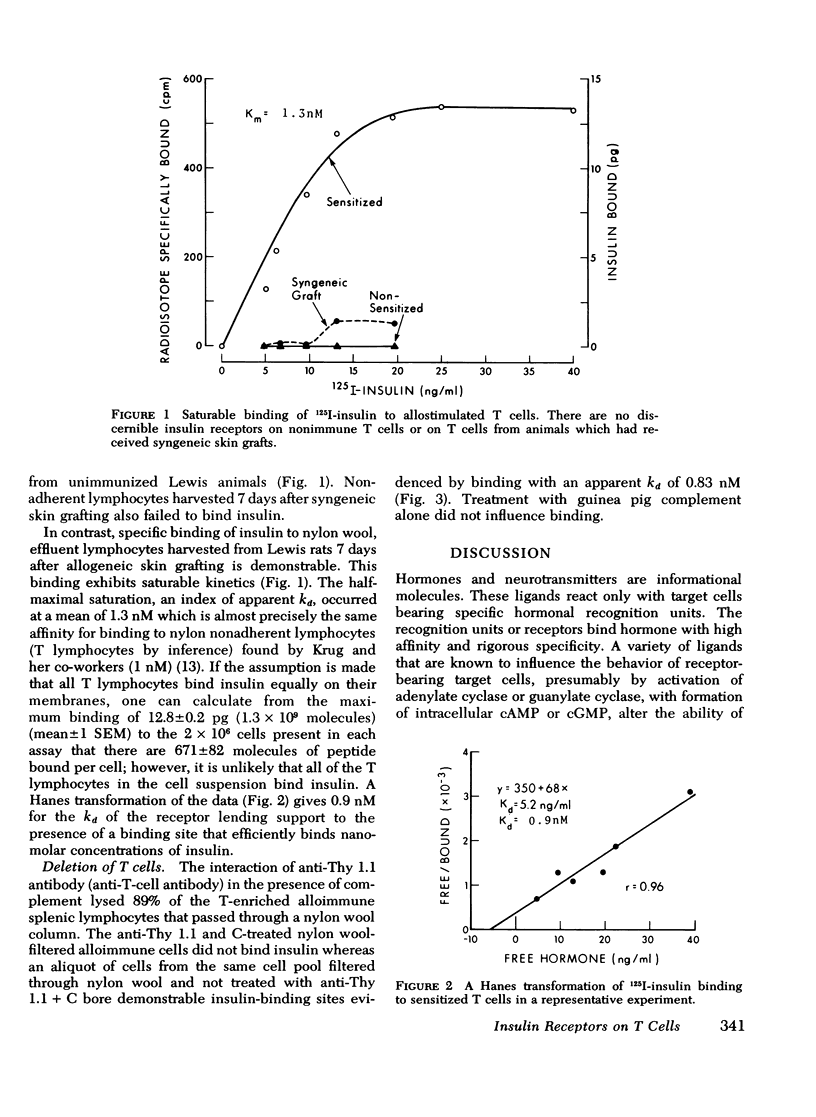
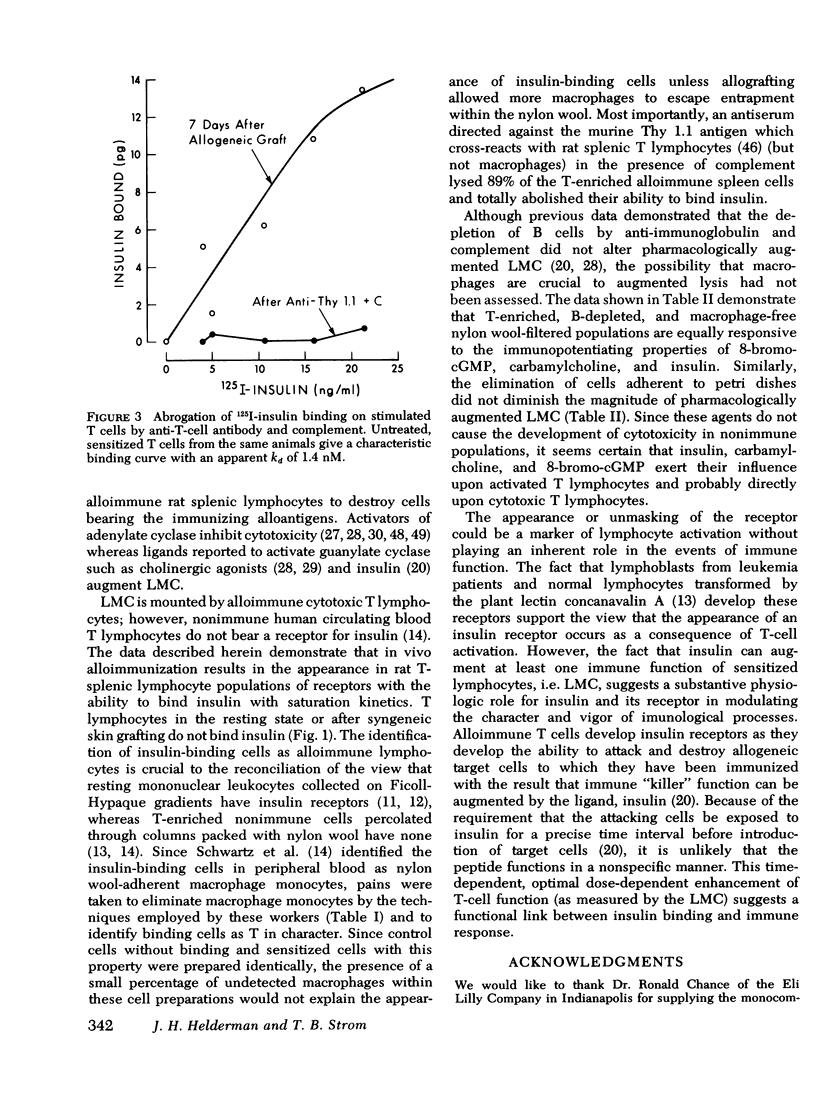


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Gavin J. R., 3rd, Lesniak M. A., Roth J. Insulin receptors in human circulating lymphocytes: application to the study of insulin resistance in man. J Clin Endocrinol Metab. 1973 Apr;36(4):627–633. doi: 10.1210/jcem-36-4-627. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970 Jul 4;227(5253):72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Desbuquois B., Krug F. Insulin-receptor interactions in liver cell membranes. Biochem Biophys Res Commun. 1971 Jul 16;44(2):333–339. doi: 10.1016/0006-291x(71)90604-8. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Unmasking of insulin receptors in fat cells and fat cell membranes. Perturbation of membrane lipids. J Biol Chem. 1971 Nov;246(21):6532–6542. [PubMed] [Google Scholar]
- Diamantstein T., Ulmer A. Effect of cyclic nucleotides on DNA synthesis in mouse lymphoid cells. Immunol Commun. 1975;4(1):51–62. doi: 10.3109/08820137509055761. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Ulmer A. Stimulation by cyclic GMP of lymphocytes mediated by soluble factor released from adherent cells. Nature. 1975 Jul 31;256(5516):418–419. doi: 10.1038/256418a0. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Jen P., Freychet P. Insulin receptors in human circulating cells and fibroblasts. Proc Natl Acad Sci U S A. 1972 Mar;69(3):747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J Exp Med. 1972 Apr 1;135(4):890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Golub E. S. The distribution of brain-associated theta antigen cross-reactive with mouse in the brain of other species. J Immunol. 1972 Jul;109(1):168–170. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Henney C. S., Lichtenstein L. M. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971 Aug;107(2):610–612. [PubMed] [Google Scholar]
- Hersey P. Thymus-dependent cytotoxic lymphocytes in the rat. Eur J Immunol. 1973 Dec;3(12):748–754. doi: 10.1002/eji.1830031203. [DOI] [PubMed] [Google Scholar]
- House P. D., Weidemann M. J. Characterization of an [125 I]-insulin binding plasma membrane fraction from rat liver. Biochem Biophys Res Commun. 1970 Nov 9;41(3):541–548. doi: 10.1016/0006-291x(70)90046-x. [DOI] [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Krug U., Krug F., Cuatrecasas P. Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed M. R., Adams L. R., Zimring A., Murnick J. G., Mayer K. Preliminary evaluation of acridine orange as a vital stain for automatic differential leukocyte counts. Am J Clin Pathol. 1972 Jan;57(1):95–102. doi: 10.1093/ajcp/57.1.95. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Pastan I., Roth J., Macchia V. Binding of hormone to tissue: the first step in polypeptide hormone action. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1802–1809. doi: 10.1073/pnas.56.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Hatfield S. Activation of purified human thymus-derived (T) cells by mitogens. II. Monocyte- macrophage potentiation of mitogen-induced DNA synthesis. J Immunol. 1976 Feb;116(2):357–362. [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulillou J. P., Carpenter C. B., d'Apice A. J., Strom T. B. The role of nonclassical Fc receptor-associated, Ag-B antigens (Ia) in rat allograft enhancement. J Exp Med. 1976 Feb 1;143(2):405–421. doi: 10.1084/jem.143.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Bear R. A., Carpenter C. B. Insulin-induced augmentation of lymphocyte-mediated cytotoxicity. Science. 1975 Mar 28;187(4182):1206–1208. doi: 10.1126/science.163492. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Deisseroth A., Morganroth J., Carpenter C. B., Merrill J. P. Alteration of the cytotoxic action of sensitized lymphocytes by cholinergic agents and activators of adenylate cyclase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2995–2999. doi: 10.1073/pnas.69.10.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Sytkowski A. J., Carpenter C. B., Merrill J. P. Cholinergic augmentation of lymphocyte-mediated cytotoxicity. A study of the cholinergic receptor of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1330–1333. doi: 10.1073/pnas.71.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Tilney N. L., Carpenter C. B., Busch G. J. Identity and cytotoxic capacity of cells infiltrating renal allografts. N Engl J Med. 1975 Jun 12;292(24):1257–1263. doi: 10.1056/NEJM197506122922402. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Segal S., Melmon K. L. Specific mitogenic activity of 8-Br-guanosine 3',5'-monophosphate (Br-cyclic GMP) on B lymphocytes. J Immunol. 1975 Jul;115(1):112–117. [PubMed] [Google Scholar]


