Abstract
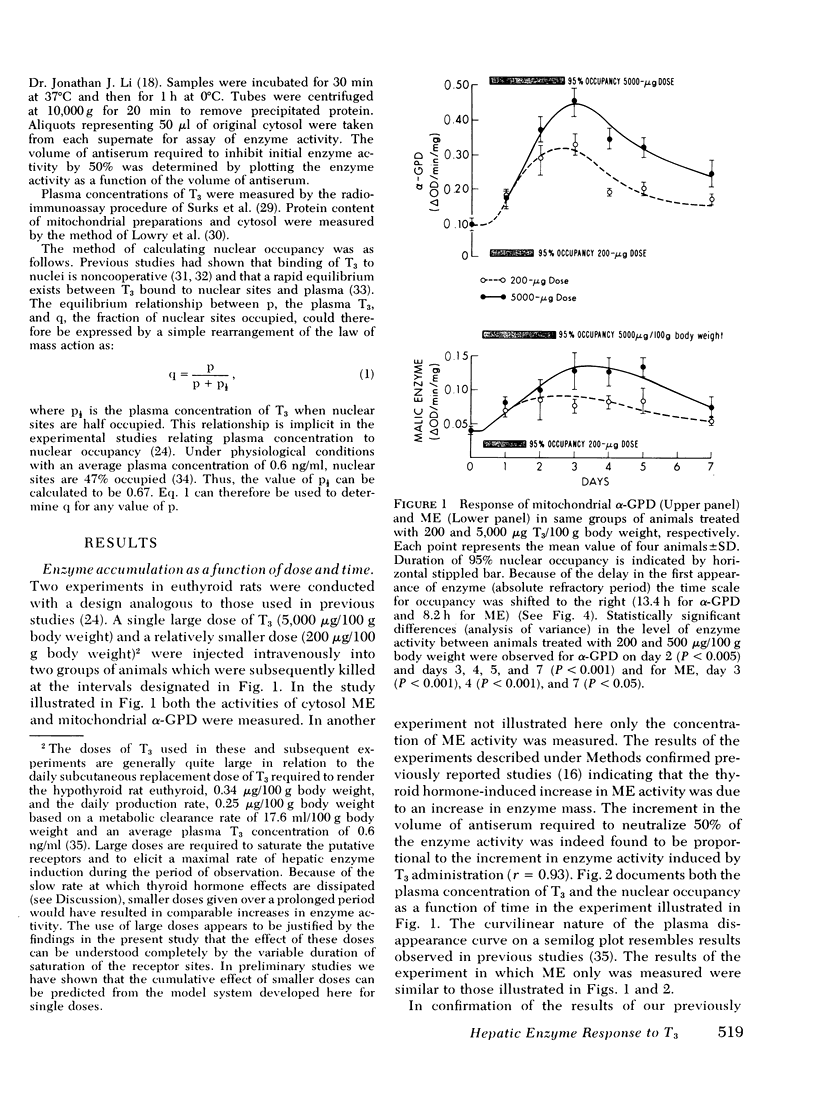
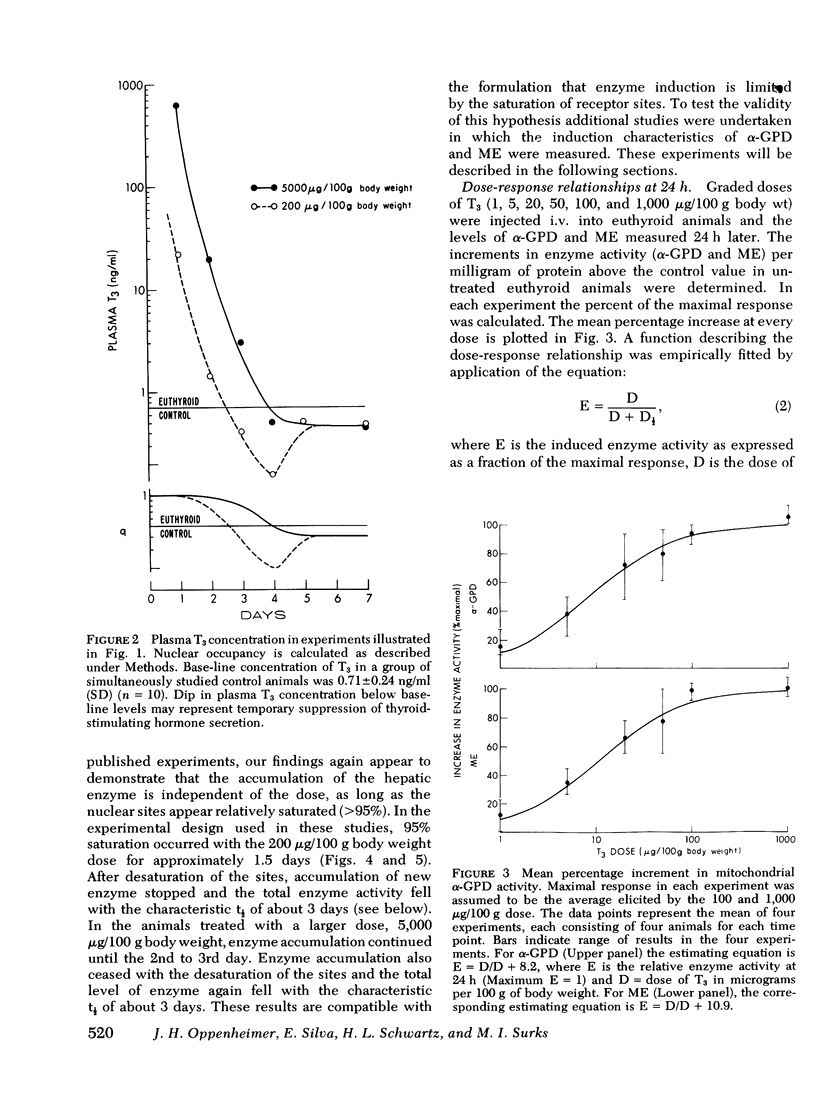
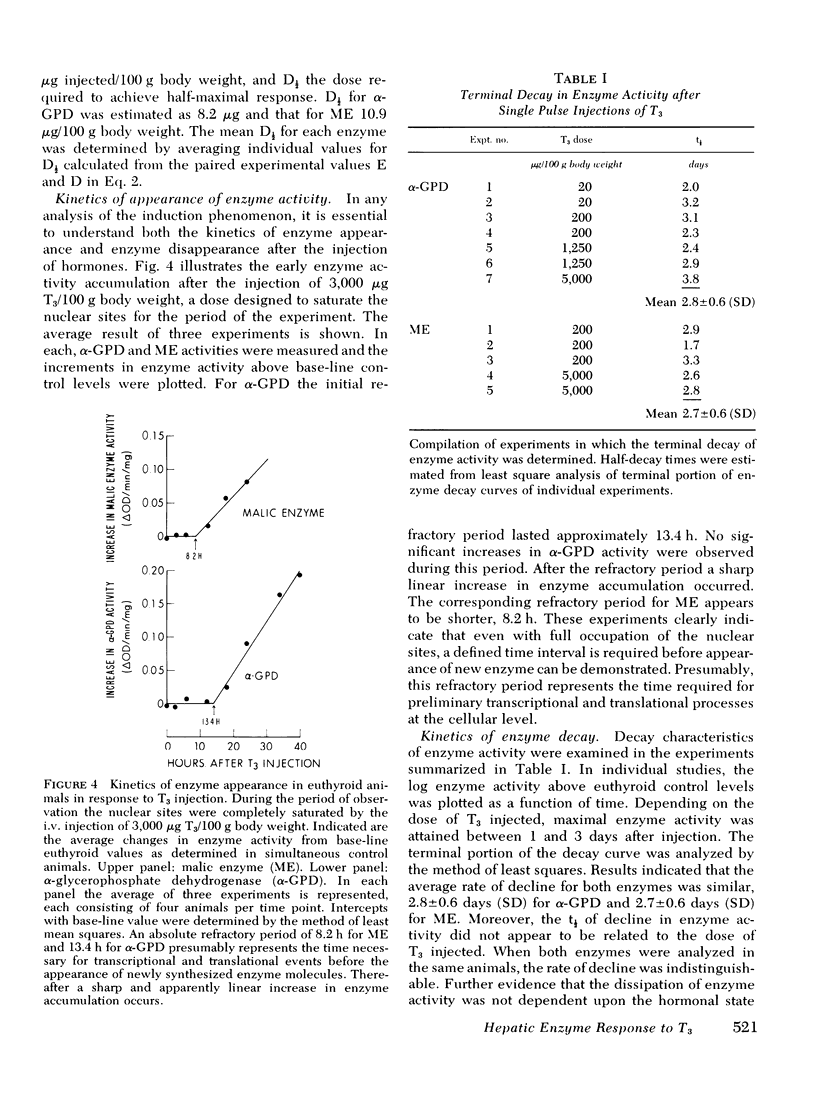
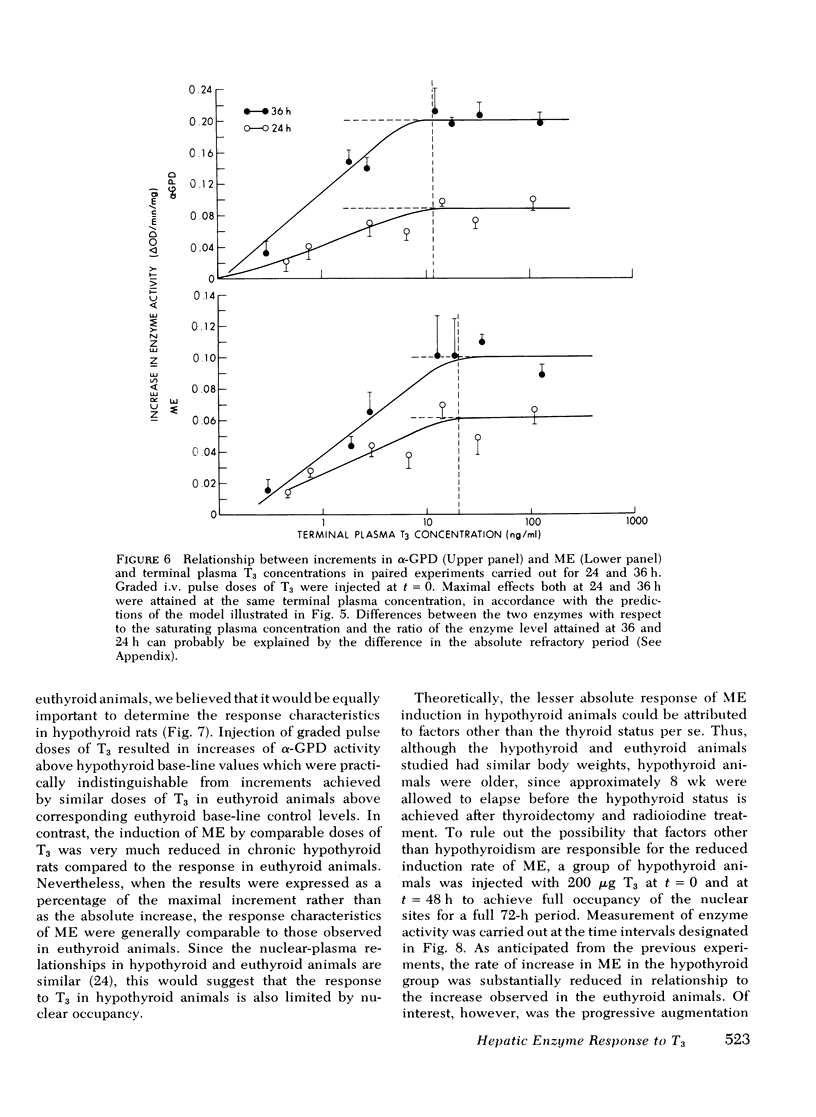
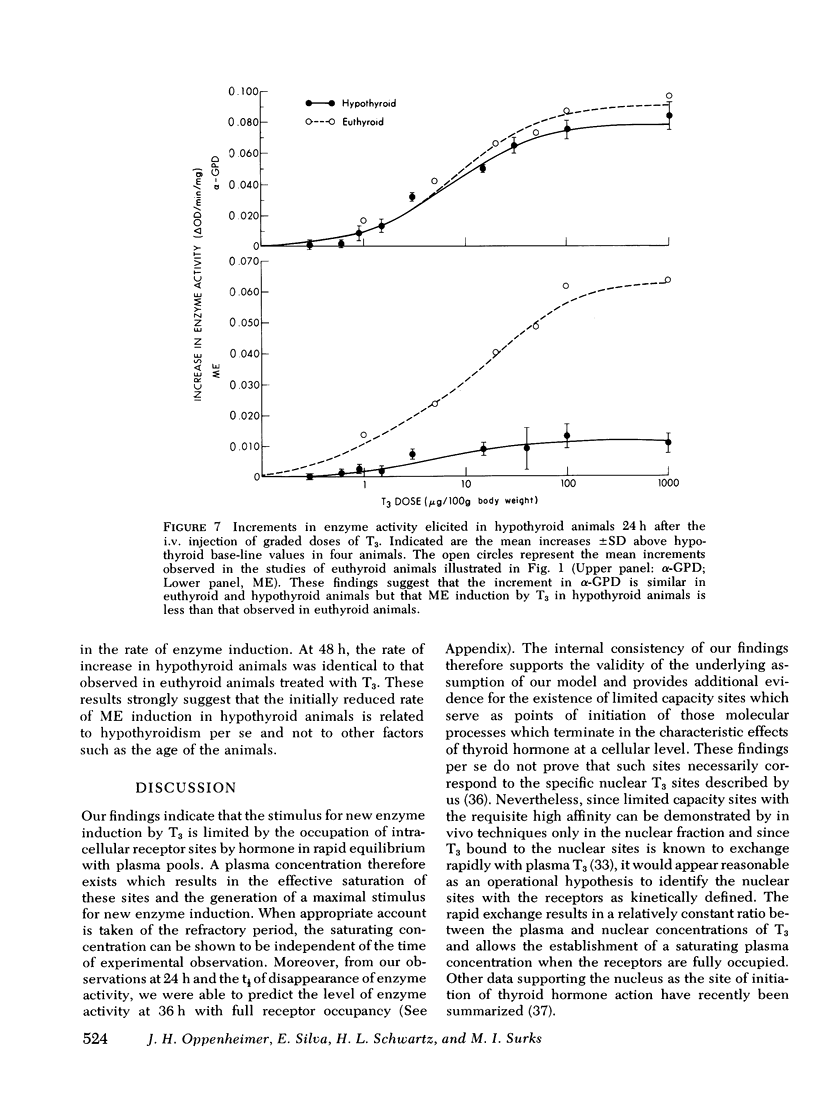
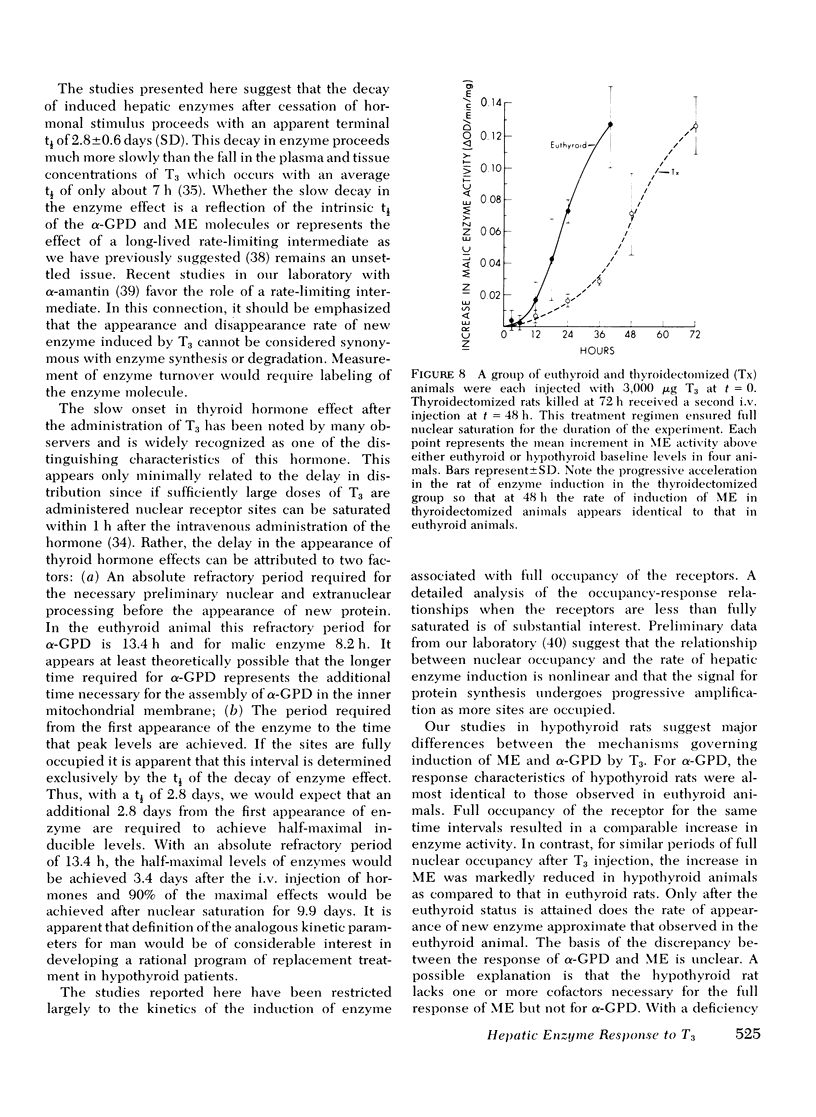
Experiments were designed to analyze the relationship of a single i.v. dose of triiodothyronine (T3), the level of plasma and hepatic nuclear T3 attained, and the tissue response as reflected in increased activity of hepatic mitochondrial alpha-glycerophosphate dehydrogenase (alpha-GPD) and cytosol "malic enzyme" (ME). These studied were carried out in euthyroid rats by varying the dose of T3 injected and the time at which the animals were killed and the enzyme levels measured. The plasma T3 concentration was determined and the fraction of nuclear sites occupied at any time t was calculated from the known plasma:nuclear relationship. As a first step, the analysis was confined to the limiting situation in which all nuclear sites were effectively saturated. The following additional information was required and obtained: A proportional relationship between the half-neutralizing volume of a specific antiserum to malic enzyme and the activity of malic enzyme was established, thus confirming previous reports that the increase in enzyme activity induced by T3 is due to increased enzyme mass. The absolute refractory period immediately after i.v. injection of T3, during which no enzyme response could be detected, was determined. This was shown to be 13.4 h for alpha-GPD and 8.2 h for ME. Lastly, the t1/2 of the enzyme decay after pulse injection of T3 was measured. This was similar for both enzymes, 2.8+/-0.6 (SD) days for alpha-GPD and 2.7+/-0.6 (SD) days for ME. The results of these studies indicated that the extent of hepatic response appears limited by full occupancy of a set of intracellular receptor sites by T3 which is in rapid equilibrium with the plasma hormone pool. The kinetic properties of the receptors, as functionally defined in these studies, resemble those associated with the recently described specific nuclear T3 sites. These data per se are thus compatible with but do not prove a nuclear site of initiation of hormone effect. Thye do allow the development of an internally consistent mathematical model which permits prediction of enzyme response when the receptor sites are fully occupied for a given length of time after the i.v. injection of hormone. A separate series of studies was carried out in thyroidectomized rats. The response characteristics of alpha-GPD were similar to those observed in euthyroid animals. In contrast, however, the early response of ME to pulse injections of T3 was very much reduced in hypothyroid animals as compared to euthryoid animals in which nuclear sites were saturated for comparable periods. These findings raise the possibility that a factor required for the induction of malic enzyme but not alpha-GPD is deficient in the hypothyroid state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodridge A. G. Hormonal regulation of the activity of the fatty acid synthesizing system and of the malic enzyme concentration in liver cells-1,2. Fed Proc. 1975 Feb;34(2):117–123. [PubMed] [Google Scholar]
- Goslings B., Schwartz H. L., Dillmann W., Surks M. I., Oppenheimer J. H. Comparison of the metabolism and distribution of L-triiodothyronine and triiodothyroacetic acid in the rat: a possible explanation of differential hormonal potency. Endocrinology. 1976 Mar;98(3):666–675. doi: 10.1210/endo-98-3-666. [DOI] [PubMed] [Google Scholar]
- Hemon P. L'activité alpha-glycérophosphate oxydase du foie de rat au cours du développement. Biochim Biophys Acta. 1967 Jan 11;132(1):175–178. doi: 10.1016/0005-2744(67)90203-3. [DOI] [PubMed] [Google Scholar]
- Hemon P. Malate dehydrogenase (decarboxylating) (NADP) and alpha-glycerophosphate oxidase in the developing rat. Biochim Biophys Acta. 1968 Mar 25;151(3):681–683. doi: 10.1016/0005-2744(68)90016-8. [DOI] [PubMed] [Google Scholar]
- Koerner D., Surks M. I., Oppenheimer J. H. In vitro demonstration of specific triiodothyronine binding sites in rat liver nuclei. J Clin Endocrinol Metab. 1974 Apr;38(4):706–709. doi: 10.1210/jcem-38-4-706. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- LEE Y. P., TAKEMORI A. E., LARDY H. Enhanced oxidation of alpha-glycerophosphate by mitochondria of thyroid-fed rats. J Biol Chem. 1959 Nov;234:3051–3054. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. L., Miller O. N. Studies on triiodothyronine-induced synthesis of liver mitochondrial alpha-glycerophosphate dehydrogenase in the thyroidectomized rat. Mol Pharmacol. 1967 Jan;3(1):44–51. [PubMed] [Google Scholar]
- Lee K. L., Sellinger O. Z., Miller O. N. Induction of mitochondrial alpha-glycerophosphate dehydrogenase by thyroid stimulating hormone and thyroid hormone analogues. Proc Soc Exp Biol Med. 1967 Oct;126(1):169–173. doi: 10.3181/00379727-126-32393. [DOI] [PubMed] [Google Scholar]
- Li J. J., Ross C. R., Tepperman H. M., Tepperman J. Nicotinamide adenine dinucleotide phosphate-malic enzyme of rat liver. Purification, properties, and immunochemical studies. J Biol Chem. 1975 Jan 10;250(1):141–148. [PubMed] [Google Scholar]
- Murphy G., Walker D. G. Enzyme synthesis in the regulation of hepatic "malic" enzyme activity. Biochem J. 1974 Oct;144(1):149–160. doi: 10.1042/bj1440149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Nuclear binding capacity appears to limit the hepatic response to L-triiodothyronine (T3). Endocr Res Commun. 1975;2(4-5):309–325. doi: 10.1080/07435807509089004. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Surks M. I., Schwartz H. L. Slow fractional removal of nonextractable iodine from rat tissue after injection of labeled L-thyroxine and 3,5,3'-triiodo-L-thyronine. A possible clue to the mechanism of initiation and persistence of hormonal action. J Clin Invest. 1972 Nov;51(11):2796–2807. doi: 10.1172/JCI107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUEGAMER W. R., WESTERFELD W. W., RICHERT D. A. ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE RESPONSE TO THYROXINE IN THYROIDECTOMIZED, THIOURACIL-FED AND TEMPERATURE-ADAPTED RATS. Endocrinology. 1964 Dec;75:908–916. doi: 10.1210/endo-75-6-908. [DOI] [PubMed] [Google Scholar]
- Ruegamer W. R., Newman G. H., Richert D. A., Westerfeld W. W. Specificity of the alpha-glycerophosphate dehydrogenase and malic enzyme response to thyroxine. Endocrinology. 1965 Oct;77(4):707–715. doi: 10.1210/endo-77-4-707. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., LEE K. THE INDUCTION OF MITOCHONDRIAL ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE BY THYROID HORMONE: EVIDENCE FOR ENZYME SYNTHESIS. Biochim Biophys Acta. 1964 Sep 11;91:183–186. doi: 10.1016/0926-6550(64)90189-6. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Percin C. J. Thyroid hormone induction of alpha-glycerophosphate dehydrogenase in rats of different ages. Endocrinology. 1966 Dec;79(6):1075–1078. doi: 10.1210/endo-79-6-1075. [DOI] [PubMed] [Google Scholar]
- Short S. H., Ruegamer W. R. Effects of route of administration and dietary hemoglobin on the liver mitochondrial alpha-glycerophosphate dehydrogenase response to various thyroxine analogs. Endocrinology. 1966 Jul;79(1):90–96. doi: 10.1210/endo-79-1-90. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Surks M. I., Koerner D. H., Oppenheimer J. H. In vitro binding of L-triiodothyronine to receptors in rat liver nuclei. Kinectics of binding, extraction properties, and lack of requirement for cytosol proteins. J Clin Invest. 1975 Jan;55(1):50–60. doi: 10.1172/JCI107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Oppenheimer J. H. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972 Dec;51(12):3104–3113. doi: 10.1172/JCI107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. PATTERNS OF DIETARY AND HORMONAL INDUCTION OF CERTAIN NADP-LINKED LIVER ENZYMES. Am J Physiol. 1964 Feb;206:357–361. doi: 10.1152/ajplegacy.1964.206.2.357. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Richert D. A., Westerfeld W. W. The concurrent induction of hepatic alpha-glycerophosphate dehydrogenase and malate dehydrogenase by thyroid hormone. Biochim Biophys Acta. 1966 Aug 24;124(2):205–309. [PubMed] [Google Scholar]
- Tepperman H. M., Tepperman J. Effect of saturated fat diets on rat liver NADP-linked enzymes. Am J Physiol. 1965 Oct;209(4):773–780. doi: 10.1152/ajplegacy.1965.209.4.773. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfeld W. W., Richert D. A., Ruegamer W. R. New assay procedure for thyroxine analogs. Endocrinology. 1965 Nov;77(5):802–811. doi: 10.1210/endo-77-5-802. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Noda L. H. Studies on tyrosine residues in porcine muscle adenylate kinase. Circular dichroism spectra and chemical modification with tetranitromethane. J Biol Chem. 1976 May 25;251(10):3021–3026. [PubMed] [Google Scholar]
- Young J. W. Effects of D- and L-thyroxine on enzymes in liver and adipose tissue of rats. Am J Physiol. 1968 Feb;214(2):378–383. doi: 10.1152/ajplegacy.1968.214.2.378. [DOI] [PubMed] [Google Scholar]


