Abstract
Nitro-oleic acid (9- and 10-nitro-octadeca-9-enoic acid, OA-NO2) is an electrophilic fatty acid nitroalkene derivative that modulates gene transcription and protein function via post-translational protein modification. Nitro-fatty acids are generated from unsaturated fatty acids by oxidative inflammatory reactions and acidic conditions in the presence of nitric oxide or nitrite. Nitroalkenes react with nucleophiles such as cysteine and histidine in a variety of susceptible proteins including transient receptor potential (TRP) channels in sensory neurons of the dorsal root and nodose ganglia. The present study revealed that OA-NO2 activates TRP channels on afferent nerve terminals in the urinary bladder and thereby increases bladder activity. The TRPV1 agonist capsaicin (CAPS, 1 μM) and the TRPA1 agonist allyl isothiocyanate (AITC, 30 μM), elicited excitatory effects in bladder strips, increasing basal tone and amplitude of phasic bladder contractions (PBC). OA-NO2 mimicked these effects in a concentration-dependent manner (1 μM – 33 μM). The TRPA1 antagonist HC3-030031 (HC3, 30 μM) and the TRPV1 antagonist diaryl piperazine analogue (DPA, 1 μM), reduced the effect of OA-NO2 on phasic contraction amplitude and baseline tone. However, the non-selective TRP channel blocker, ruthenium red (30 μM) was a more effective inhibitor, reducing the effects of OA-NO2 on basal tone by 75% and the effects on phasic amplitude by 85%. In bladder strips from CAPS-treated rats, the effect of OA-NO2 on phasic contraction amplitude was reduced by 65% and the effect on basal tone was reduced by 60%. Pretreatment of bladder strips with a combination of neurokinin receptor antagonists (NK1 selective antagonist, CP 96345; NK2 selective antagonist, MEN 10,376; NK3 selective antagonist, SB 234,375, 1 μM each) reduced the effect of OA-NO2 on basal tone, but not phasic contraction amplitude. These results indicate that nitroalkene fatty acid derivatives can activate TRP channels on CAPS-sensitive afferent nerve terminals, leading to increased bladder contractile activity. Nitrated fatty acids produced endogenously by the combination of fatty acids and oxides of nitrogen released from the urothelium and/or afferent nerves may play a role in modulating bladder activity.
Introduction
Nitro-oleic acid (OA-NO2) is one of a family of electrophilic nitroalkenyl fatty acids formed by reactions between unsaturated fatty acids, nitric oxide (NO)- and nitrite (NO2 -)-derived nitrogen dioxide (.NO2) that are promoted by the pro-oxidative condtions of inflammation. Also, physiological conditions where pH is low enough (<pH 6) to protonate NO2 - to nitrous acid (HNO2) will yield the proximal nitrating species .NO2 (Khoo, et al., 2010). Nitro-fatty acids are present in healthy human urine and are produced at increased levels during metabolic stress and inflammatory conditions, being detected at nM to μM concentrations in vivo (Baker, et al., 2005, Rudolph and Freeman, 2009, Rudolph, et al., 2010). Because of a potent and reversible electrophilic reactivity, it has recently been appreciated that electrophilic lipid derivatives can mediate transcriptional regulatory actions that overall result in anti-inflammatory responses (Rudolph and Freeman, 2009). Specifically, OA-NO2 and other nitro-fatty acids can activate PPARγ receptors (Baker, et al., 2005), inhibit LPS-induced cytokine expression (Wang, et al., 2010), induce HO-1 expression via activation of Nrf2-regulated gene expression (Khoo, et al., 2010) and inhibit NF-κB signaling (Cui, et al., 2006). OA-NO2 protects against LPS-induced endotoxemia and multi-organ failure in mice (Wang, et al., 2010), attenuates renal ischemia/reperfusion injury in mice, likely via inhibition of the inflammatory response (Liu, et al., 2008) and reduces ischemia/reperfusion injury in a murine model of focal cardiac ischemia (Rudolph, et al., 2010). Therefore, the impact of nitro-fatty acids in models of inflammation and metabolic stress is a subject requiring further investigation (Baker, et al., 2009).
OA-NO2 activates TRPV1 and TRPA1 channels in sensory neurons (Sculptoreanu, et al., 2010, Taylor-Clark, et al., 2009). These channels play an important role in mediating neurogenic inflammation and pain induced by noxious chemicals or thermal stimuli, TRPA1 channels have ankyrin-like repeats in the N terminus that are rich in cysteine residues (Bandell, et al., 2007, Macpherson, et al., 2007) and TRPV1 channels have extracellular cysteines (Jin, et al., 2004, Susankova, et al., 2006) that react with electrophiles and other thiol modifying species via Michael addition to alter channel gating and excitability (Bandell, et al., 2004, Macpherson, et al., 2007, Salazar, et al., 2008). It has been proposed that OA-NO2 covalently modifies the negatively charged cysteine of TRP channels leading to changes in channel function (Rudolph and Freeman, 2009).
By activating TRPV1 and TRPA1 channels, OA-NO2 induces calcium influx, depolarization and firing (Sculptoreanu, et al., 2010, Taylor-Clark, et al., 2009). Additionally, high concentrations of OA-NO2 suppress the generation of firing in dorsal root ganglion (DRG) neurons, another mechanism that could contribute to anti-inflammatory effects.
TRP channels are expressed in bladder afferent nerves and are involved in bladder pain, inflammation and overactivity (Andersson, et al., 2010, Everaerts, et al., 2008). Activation of TRPV1 or TRPA1 channels in bladder afferent nerves also triggers the release of neurokinins that can act on nerves, urothelium or bladder smooth muscle to modulate bladder function (Everaerts et al., 2008; Gappetti et al., 2008). Herein we evaluate the effects of OA-NO2 on contractile activity of muscle strips of rat urinary bladder to determine if OA-NO2 can activate TRPV1 or TRPA1 channels in afferent nerves. The results indicate that OA-NO2 increases bladder strip activity via activation of TRP channels and release of neurokinins from capsaicin-sensitive afferent nerves.
Methods
Bladder strip preparation: All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Bladder strips from adult female (200 – 250g) Sprague Dawley rats were prepared as described previously (Artim, et al., 2009). Briefly, the bladder was removed from isoflurane (4 % in O2) anesthetized rats, placed in warm Krebs solution (composition in mM: NaCl 118, KCl 4.7, CaCl2 1.9, MgSO4 1.2, NaHCO3 24.9, KH2PO4 1.2, dextrose 11.7; pH. 7.4 bubbled with 95% O2, 5% CO2) and cut into four to eight longitudinal strips (~1.5 mm × 8-10 mm). Strips were tied with fine thread at each end, mounted in a vertical double jacketed organ bath in oxygenated Krebs solution (15 ml volume) and kept at 37° C via a circulating warm water bath. Tissue was stretched by applying baseline tension of 10 mN (1 g) and allowed to equilibrate for 1-2 h prior to drug testing.
To determine the concentration-response relationship of OA-NO2, each strip was tested with only one concentration due to the possibility of desensitization. The effects of antagonists on the OA-NO2 response were tested using a concentration of OA-NO2 (15 μM) that did not induce desensitization with multiple applications (Fig. 1 D and E). Antagonists were applied to bladder strips after the first application of OA-NO2 and 15-20 min prior to the second application.
Figure 1.
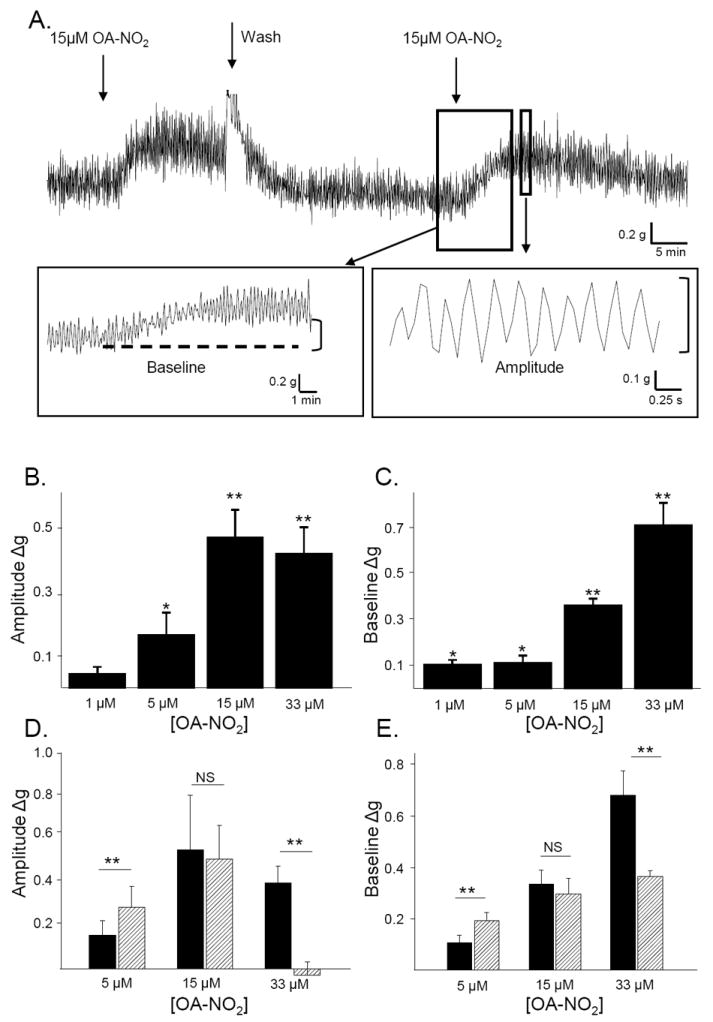
A. OA-NO2 (15μM, arrows) increases the amplitude of phasic contractions and baseline tone of bladder strips. Insets illustrate methods for the measurement of changes in bladder activity. The baseline was measured as the average of minimum points. The amplitude of phasic contractions was measured as the difference between the minimum and maximum of each peak. Contractions and baseline were measured in 3 min intervals before drug application and at the time of maximal drug effect. Summary data plotting the concentration-dependent effect of OA-NO2 on phasic contraction amplitude (B) and baseline tone (C). Each strip was treated with just one concentration of OA-NO2. Calibration bars refer to time (sec) and tension (grams). ** p < 0.01, * p < 0.05 by one-sample t-test. n = 5 to 12 strips for each concentration. Summary data comparing the effects of the 1st (black bars) and 2nd application (hatched bars) of OA-NO2 on phasic contraction amplitude (D) and baseline tone (E). ** p < 0.01*, p < 0.05, NS p > 0.05 by paired t-test. n = 5 to 8 strips for each concentration.
Capsaicin pretreatment
In some experiments designed to examine the role of capsaicin-sensitive, C-fiber afferent nerves in the effects of OA-NO2, animals were pretreated with capsaicin (125 mg/kg subcutaneous injection; dissolved in 10% ethanol, 10% Tween 80 and 80% physiological saline) to desensitize afferent nerves. Capsaicin was administered under isoflurane anesthesia (3.5% in O2) in three injections (25, 50 and 50 mg/kg) over a two day period (at ~12 h intervals). The bladders were removed and used for experiments 4 days after the last injection.
Chemicals
Chemicals used in this study include: nitro-oleic acid (OA-NO2); oleic acid a non-nitrated fatty acid, diarylpiperazine analogue, (DPA) a selective TRPV1 receptor antagonist (Ki = 6 nM for inhibition of acid pH evoked responses and 35 nM for inhibition of CAPS evoked responses) (Valenzano, et al., 2003), a gift from Neurogen (CT, USA), HC3-03001 (HC3), a selective TRPA1 receptor antagonist (Hydra Biosciences, Inc., Cambridge, MA), ruthenium red (RR): non-selective TRP channel antagonist, capsaicin (CAPS) a TRPV1 channel agonist; allyl isothiocyanate (AITC) a TRPA1 channel agonist, CP 96345: an NK1 receptor antagonist, MEN 10,376: an NK2 receptor antagonist, SB 234,375, an NK3 receptor antagonist, tetrodotoxin (TTX): a voltage-gated sodium channel blocker and nifedipine (NIF) an L-type calcium channel antagonist. OA-NO2 was synthesized as described previously (Baker, et al., 2005). All other agents were obtained from Sigma Aldrich (St. Louis, MO). Vehicles (0.1% ethanol for OA-NO2 and 0.1% DMSO for CAPS and AITC) had no effect on bladder strip contractions.
Data analysis
All data are presented as mean ± S.E.M. Bladder strip activity was measured in 5 min intervals immediately before and 10-20 min after drug application, at the time when the drug had exerted its maximal effect. The effects of drugs were measured as change in baseline tone or change in amplitude or frequency of phasic contractions that were ≥ 0.1 g (Fig. 1A). Data are reported as absolute change in tension (g). Statistical significance was tested with one-sample t-test, or paired two-tailed t-test with layered Bonferroni post hoc test for multiple comparisons when appropriate using Excel and Origin 7 software. Data were considered statistically significant when p < 0.05.
Results
OA-NO2 stimulates phasic activity in rat bladder strips
When stretched under 1 g tension, bladder strips displayed phasic contractions with an average amplitude of 0.75±0.1 g and frequency of 4.3±0.1 / min, (n = 73). Application of nitro-oleic acid (OA-NO2; 1-33 μM) produced a concentration-dependent increase in baseline tone and in the amplitude of phasic contractions, but did not alter contraction frequency (Fig. 1). The threshold concentration for eliciting an increase in phasic contraction amplitude was greater (5 μM) than that for increasing baseline tone (1 μM) (Fig. 1B and C). The effect of OA-NO2 was reversible after washout and repeatable. The effect of the first and second applications of OA-NO2 (15 μM) on either phasic contraction amplitude or baseline tone were not significantly different (Fig. 1 D and E). Therefore, 15 μM OA-NO2 was used for experiments in which the effects of antagonists were tested on the response to repeated applications of OA-NO2 in the same bladder strip. Repeated applications of a higher concentration of OA-NO2 (33 μM) produced marked desensitization; such that a second OA-NO2 application at a 15-20 min interval elicited a significantly smaller effect (54% of the first application) on baseline tone and no effect on phasic contraction amplitude (Fig. 1 D and E). Oleic acid (OA, a native fatty acid) did not alter phasic activity or baseline tone at concentrations up to 33 μM (data not shown).
OA-NO2 targets TRP channels
The effects of OA-NO2 were mimicked by application of a TRPV1-selective agonist (capsaicin; CAPS) or a TRPA1-selective agonist (allyl isothiocyanate; AITC). Application of CAPS (1 μM, n = 22) or AITC (30 μM, n = 23) increased the amplitude of phasic contractions and baseline tone (Fig. 2), but did not alter the frequency of phasic contractions. When agonist-induced contractions were normalized to contractions elicited by KCl (80 mM), CAPS elicited a larger response than AITC and OA-NO2, which produced phasic contractions of similar relative amplitude (Fig. 2D), and similarly increased baseline tone (Fig. 2E). The effect of CAPS was rapid in onset, reaching a peak increase in baseline tone within a min and a peak increase in phasic contraction amplitude within 2-3 min after application. The effects of OA-NO2 or AITC were slower; the effect on baseline tone and phasic contraction amplitude peaking 3-5 min after application.
Figure 2.
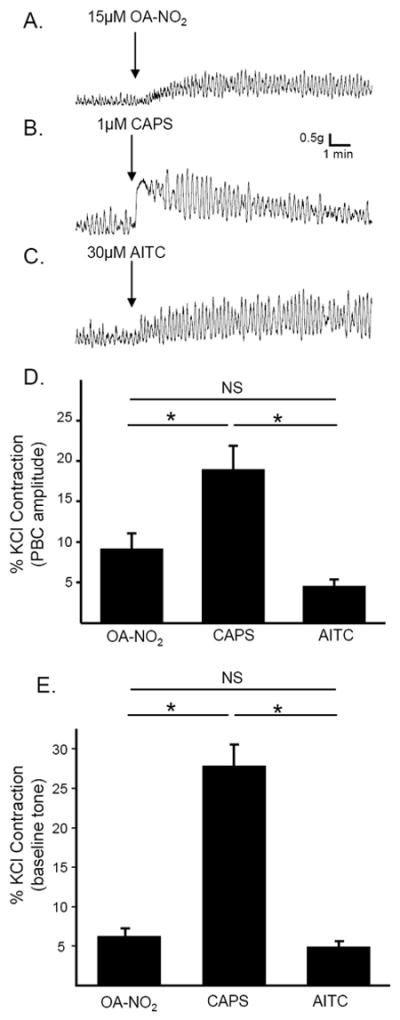
Activation of TRPV1 or TRPA1 receptors mimics the effects of OA-NO2. Examples of agonist-induced phasic contractions in rat bladder strips; arrows indicate time of application of OA-NO2 (15μM; A.), CAPS (1μM; B.) and AITC (30μM; C.) D. Summary data showing the amplitude of agonist-induced increase in phasic contraction amplitude (D) or baseline tone (E) normalized to KCl-evoked contraction. * p < 0.05, NS p > 0.05 by one-way ANOVA, n = 9 to 13.
The TRPV1 antagonist, a diaryl piperazine analogue (DPA) in a concentration (1 μM) shown previously to block the effects of CAPS (1 μM), (Sculptoreanu, et al., 2010) prevented the effect of CAPS on phasic contraction amplitude and substantially reduced the effect of CAPS on baseline tone by 92.8% (p<0.01, n=12, Fig. 3 A, C and D). The TRPA1 antagonist HC3 in a concentration (30 μM) shown previously to block the effects of AITC on calcium influx in DRG neurons (Sculptoreanu, et al., 2010), significantly decreased the effect of AITC on phasic contraction amplitude (by 54.4%, p < 0.05) but only partially decreased the effect of AITC on baseline tone (30.6%, p > 0.05, n =13, Fig. 3 B, C and D).
Figure 3.
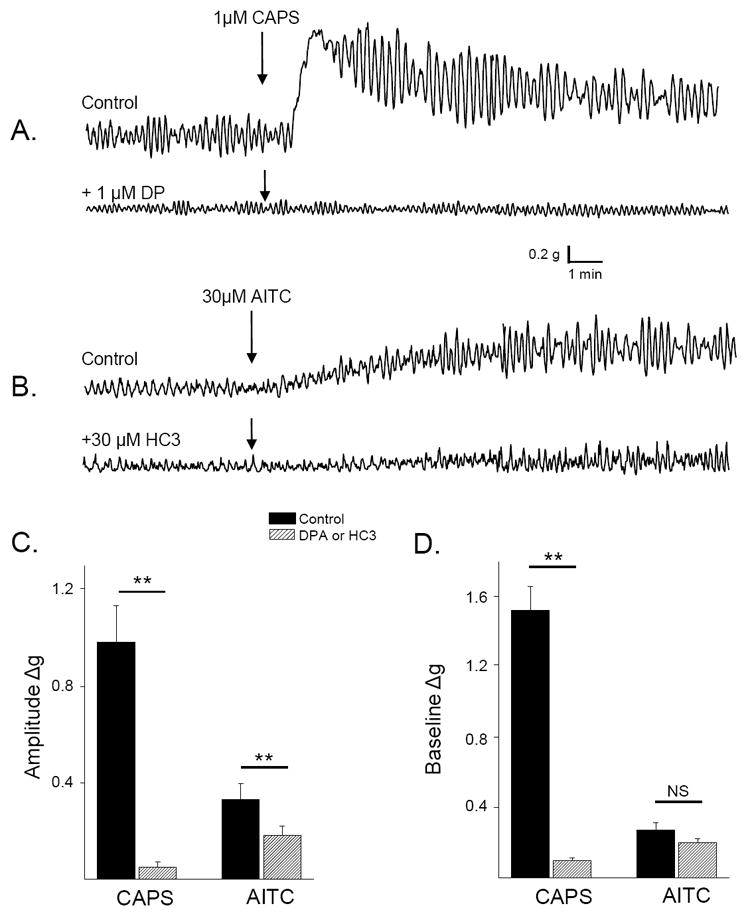
Effects of selective TRPV1 and TRPA1 agonists and antagonists on activity of rat bladder strips. Arrows indicate time of drug application. CAPS (1μM; A.) and AITC (30uM; B.) elicit increases in phasic contractions and baseline tone that are blocked by a TRPV1-selective antagonist (DPA; 1μM) and by a TRPA1-selective antagonist (HC3; 30μM), respectively. Antagonists were applied 15-20 min before agonists. Examples are from four different strips. Summary data of the effect of CAPS (1μM) and AITC (30μM) on contraction amplitude (C) and baseline tone (D) in the absence (black bars) and presence (hatched bars) of DP (1μM) or HC3 (30μM), respectively. ** p < 0.01, NS p > 0.05 by unpaired t-test, n = 12 to 23 strips.
To determine if OA-NO2 elicited responses by activating TRPV1 and/or TRPA1 channels, bladder strips were treated with DPA (1 μM), HC3 (30 μM), or a combination of both antagonists for 15-20 min prior to the application of OA-NO2. The amplitude of phasic contractions prior to the application of OA-NO2 was significantly decreased by DPA (1 μM, 20.4%), HC3 (30 μM, 11.6%) or a combination of the two antagonists (26.6%) within 15 min of application (Fig. 4, p<0.05, n = 6-12). Neither antagonist alone had an effect on baseline tone or the frequency of phasic contractions. HC3 (30 μM) significantly reduced the facilitatory effect of OA-NO2 on both phasic contraction amplitude (59%, p<0.05, n = 5) and baseline tone (44.5%, p<0.05, n=5, Fig. 5B and E). DPA (1 μM) had a similar effect, reducing the effect of OA-NO2 on phasic contraction amplitude by 39.2% (p<0.05,n=8) and on baseline tone by 52.7% (p<0.05, n=8, Fig. 5A and D). Pretreatment with a higher concentration of HC3 (60 μM) did not elicit a further reduction in the effect of OA-NO2 (n = 3 strips, data not shown). The combination of both antagonists reduced the effect of OA-NO2 on phasic contraction amplitude by 82.2% and on baseline tone by 80.0% (p<0.05, n = 9, Fig 5F).
Figure 4.
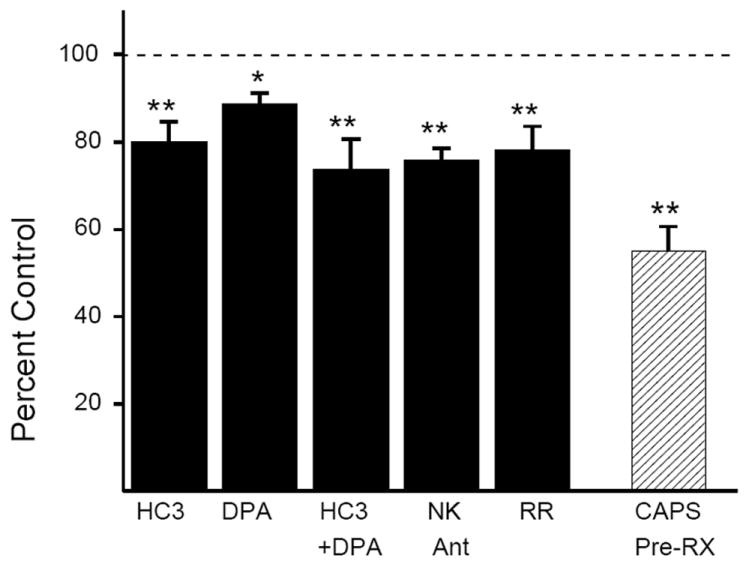
Summary data showing the effects of TRP channel antagonists (HC3 30 μM, DPA 1μM, a combination of HC3 and DPA, ruthenium red (RR, 30 μM) or NK 1,2,3 receptor antagonists (1μM each, black bars) on the amplitude of phasic contractions shown as percent of control, measured 17-20 min after drug application (black bars: ** p < 0.01, *p < 0.05, NS p > 0.05 by paired t-test, n = 6 to 12). Amplitude of phasic contractions in bladders from CAPS-pretreated rats are compared to contraction amplitude in bladders from vehicle-control treated rats. (hatched bars: ** p < 0.01, by unpaired t-test, n = 12 in each group)
Figure 5.
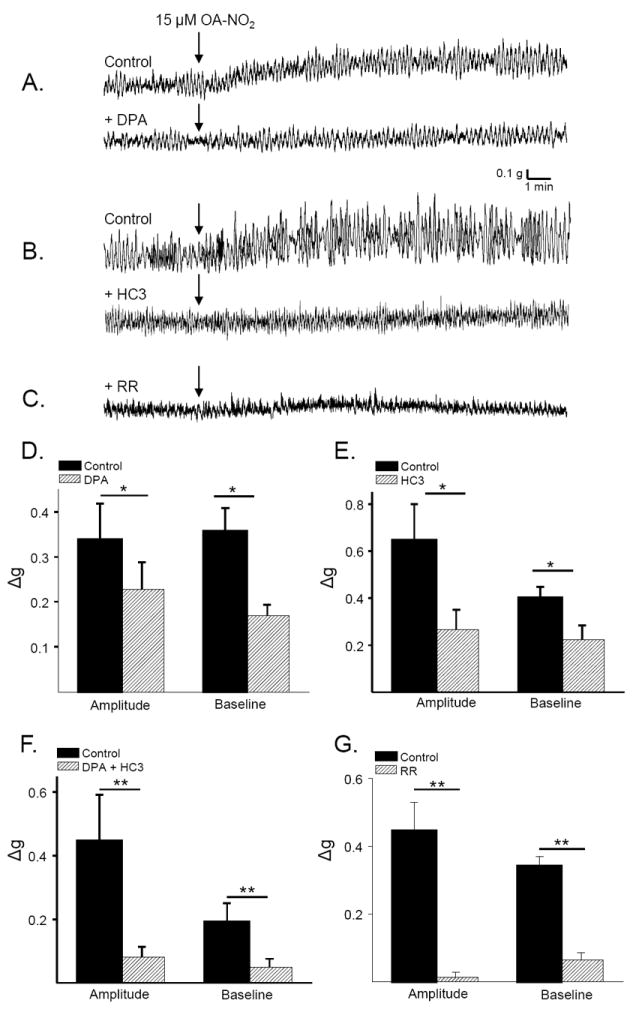
Effect of TRP channel antagonists on the response to OA-NO2. A. Increased phasic contractions and baseline tone induced by OA-NO2 (15μM; arrows) before (top trace) and after (bottom trace) application of DPA (1μM) in the same strip. B. In another strip the effects of OA-NO2 (15μM) before (top trace) and after (bottom trace) HC3 (1μM). C. The effects of OA-NO2 (15μM) after pretreatment with RR (30μM). Summary data showing the effect of OA-NO2 on contraction amplitude and baseline tone before (black bars) or after (hatched bars) application of 1μM DPA (D), 30μM HC3 (E), or a combination of both antagonists (F). * p < 0.05, by paired t-test, n = 6-9 strips. G. Summary data showing the effect of OA-NO2 on contraction amplitude and baseline tone in control strips (black bars) and in a second population of strips pretreated for 20 min with RR (30μM, hatched bars). ** p < 0.01, by unpaired t-test, p = 7 to 8 strips.
Ruthenium red (RR,30 μM), a non-selective TRP channel blocker, reduced the amplitude of phasic contractions by 22.2 ±2.8% (n = 7, Fig. 4) but had no effect on baseline tone or the frequency of phasic contractions. Pretreatment with RR (30 μM) eliminated the effect of OA-NO2 on phasic contraction amplitude and significantly reduced by 84.2% the effect on baseline tone (p < 0.01, n=7, Fig. 5 C and G). At 30 μM, RR acted selectively to block TRP channels, as it prevented the effect of CAPS (1 μM) and significantly reduced the effect of AITC (30 μM) on phasic contraction amplitude (by 63%), but did not alter carbachol- or KCl- evoked contractions (table 1). Higher concentrations of RR (up to 100 μM) were non-selective for TRP channels in our preparation, reducing the contractile response to carbachol, and KCl.
Table 1.
Effect of ruthenium red (30μM) on agonist-induced contractions
| Drug | Contraction amplitude (control) | Contraction amplitude (after 30μM RR) |
|---|---|---|
| OA-NO2 (30μM) | 0.44±0.08Δg; n = 24 | 0.01±0.02 Δg; n = 7 p<0.001 |
| CAPS (1μM) | 0.98±0.1Δg; n=22 | 0.04±0.05Δg; n = 7 p<0.001 |
| AITC (30μM) | 0.4±0.07Δg; n =19 | 0.16±0.09Δg; n = 7 p<0.01 |
| Carbachol (1μM) | 3.5±0.6g; n = 8 | 2.4± 0.3g; n = 9 NS |
| KCl (80mM) | 3.1± 0.4g; n = 23 | 2.8± 0.2g; n = 19 NS |
The effects of ruthenium red on agonist-induced contractions. Results for CAPS and AITC are expressed as the change in the amplitude of phasic contractions. The results for carbachol and KCl are expressed as the magnitude of large-amplitude coordinated contraction. n = 11 to 24.
The role of neuropeptides released from capsaicin-sensitive afferent nerves in mediating OA-NO2 effects
In one group of experiments, rats were pretreated with CAPS (125 mg/kg, (Maggi, et al., 1989)) 4 days prior to the experiment to desensitize CAPS-sensitive, afferent nerves. The basal phasic contractions in bladder strips from CAPS pretreated animals were significantly reduced (43%, p<0.05, Fig. 4) and in these strips the effect of CAPS on phasic contraction amplitude was eliminated and the effect on baseline tone reduced by 97.7% (p < 0.01, n=14, Fig. 6 B and D). In these experiments, OA-NO2 was tested in some strips after CAPS and in others before CAPS. The effects of OA-NO2 were not different in the two experiments, thus the data were pooled. In these strips, the effect of OA-NO2 on phasic contraction amplitude and baseline tone was significantly reduced (by 36.7% and 24.5%, respectively, p < 0.05, n=14, Fig. 6 A and C). In CAPS-pretreated preparations, the effect of AITC (applied either before or after OA-NO2) on phasic contraction amplitude was eliminated and the effect on baseline tone reduced by 46.4% (p < 0.05, n = 10, Fig. 6E).
Figure 6.
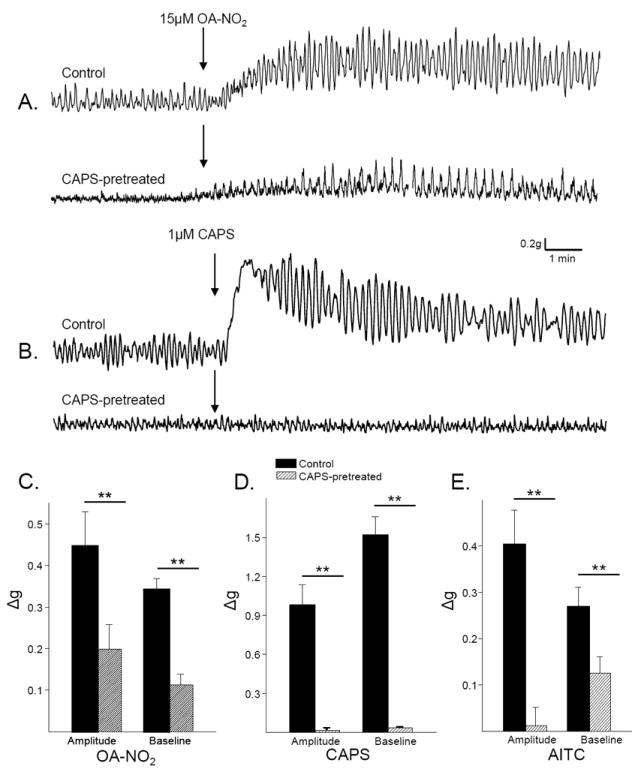
Capsaicin pretreatment reduces the effect of OA-NO2. A. OA-NO2-induced increase in phasic contractions and baseline tone in a bladder strip from a vehicle treated rat (control) and a bladder strip from a CAPS-pretreated rat. B. CAPS-induced increase in phasic contractions and baseline tone from a vehicle treated rat bladder strip (control) and a bladder strip from a CAPS-pretreated rat. Summary of the effects of OA-NO2 (C), CAPS (D) and AITC (E) on contraction amplitude and baseline tone in control bladder strips (black bars) and in bladder strips from CAPS-pretreated rats (hatched bars). * p < 0.05, ** p < 0.01, by unpaired t-test, n = 12 strips from vehicle treated rats and 12 strips from CAPS-pretreated rats. Each agonist was tested on at least 6 strips from each group.
To determine the role of neuropeptide release in the response to OA-NO2, bladder strips were treated with a combination of NK receptor 1,2 and 3 antagonists (1 μM each), which in previous experiments blocked completely the effect of substance P in bladder strips (Meini, et al., 1994). The NK receptor antagonists decreased the amplitude of phasic contractions by 24.5% prior to the application of OA-NO2 (p < 0.01, n = 7, Fig. 4) but did not alter baseline tone or the frequency of phasic contractions. Pretreatment of the bladder strips with the NK receptor antagonists significantly reduced the effect of OA-NO2 on phasic contraction amplitude by 73.2% and the effect on baseline tone by 58.3% (p < 0.01, n = 8, Fig. 7).
Figure 7.
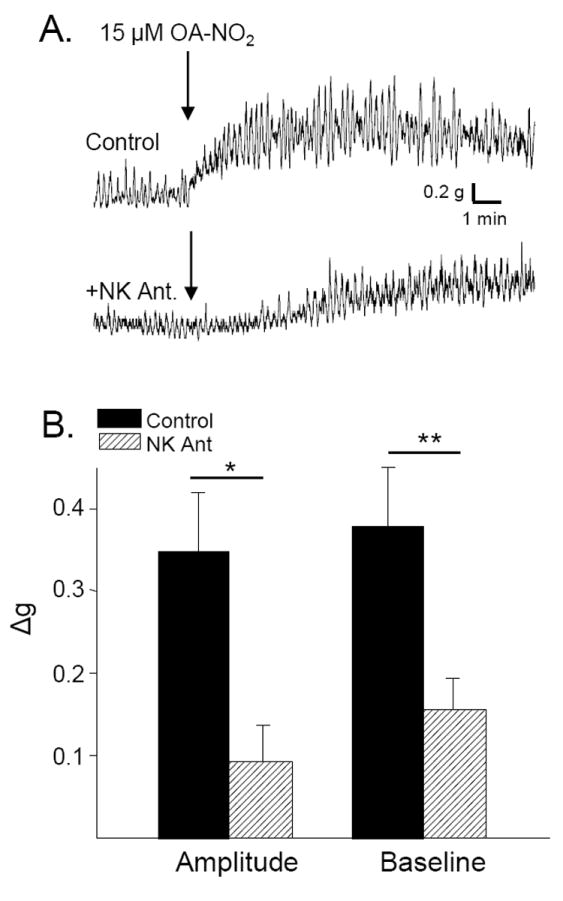
Effect of neurokinin receptor antagonists on the response to OA-NO2. A. OA-NO2-induced increase in phasic contractions and baseline tone in a bladder strip (top trace) is markedly suppressed (bottom trace) by application of a combination of NK 1,2,3 receptor antagonists (1 μM each). B. Summary data showing the effect of OA-NO2 on contraction amplitude and baseline tone before (black bars) and after (hatched bars) application of the combination of NK receptor antagonists. * p < 0.01 by paired t-test, n = 5.
Effect of TTX or an L-type calcium channel blocker on the effect of OA-NO2
Application of TTX (1 μM) had no effect on phasic activity or baseline tone in rat bladder strips (95.0±3.3% and 102.2±2.6% of control, respectively, p > 0.05, n = 8, Fig. 8A). Subsequent application of OA-NO2 (15 μM) significantly increased the amplitude of phasic contractions and baseline tone (Fig. 8 A, C and D). The effect on amplitude of phasic contractions was not different than the effect in control strips, but the effect on baseline tone was smaller than in the absence of TTX. In contrast, application of nifedipine (NIF, 10 μM) eliminated phasic activity and significantly reduced baseline tone by 45.7% (n = 8, Fig. 8B). Subsequent application of OA-NO2 (15 μM) did not induce phasic contractions, or increase baseline tone (n = 8, Fig. 8 B, C and D)
Figure 8.
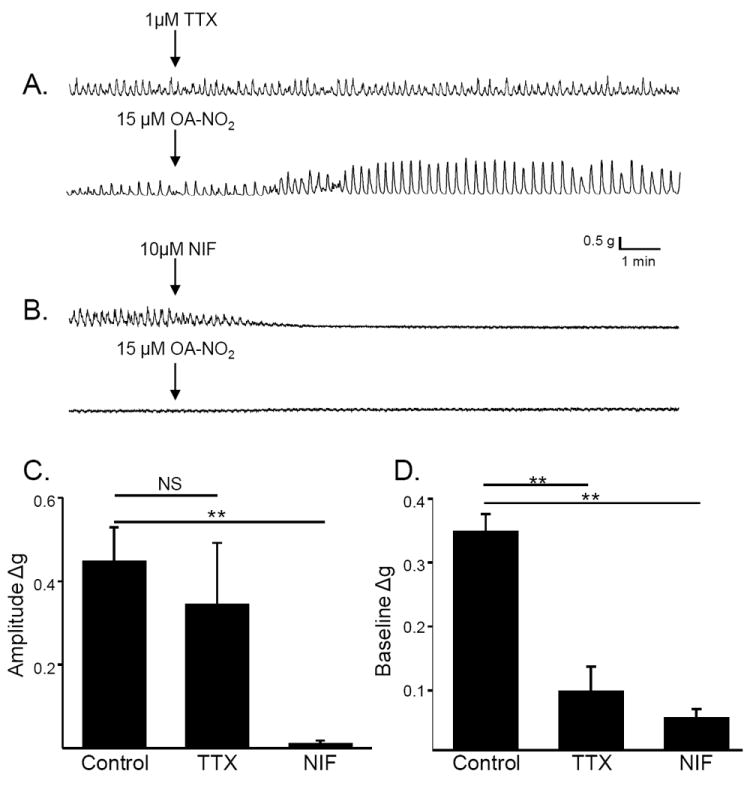
Effect of tetrodotoxin (TTX) and nifedipine (NIF) on the response to OA-NO2. A. TTX (1μM), which did not alter basal contractions or baseline tone of a bladder strip (top trace), reduced the response to a subsequent application of OA-NO2 (15μM) applied 15 min later (bottom trace). B. Nifedipine (NIF, 10 μM), which completely suppressed phasic contractions and reduced baseline tone of bladder strip (top trace), completely blocked the effect of a subsequent application of OA-NO2 (15μM) applied 15 min later (bottom trace). Summary data showing the effect of OA-NO2 on contraction amplitude (C) and baseline tone (D) in control strips and in strips pretreated for 15 min with TTX (1μM) or NIF (10μM) **p < 0.01, NS p > 0.05 by one-way ANOVA, n = 8 to 9.
Discussion
The results presented here demonstrate that low micromolar concentrations of OA-NO2, but not OA, increase phasic activity and baseline tone in rat bladder strips. Various treatments, including (1) desensitization of C-fiber afferent nerves with CAPS, (2) application of TRPV1, TRPA1 or a non-selective TRP antagonist (ruthenium red) or (3) application of a combination of NK receptor antagonists reduced or blocked the effect of OA-NO2. These data indicate that the effects of OA-NO2 were mediated in part by triggering the release of neurokinins from CAPS-sensitive bladder afferent nerves via activation of TRP channels (Fig. 9). These data raise the possibility that TRP channels may be targets for endogenous nitro-fatty acid derivatives produced during cystitis and that these agents may have a modulatory role in the generation of bladder pain and inflammation.
Figure 9.
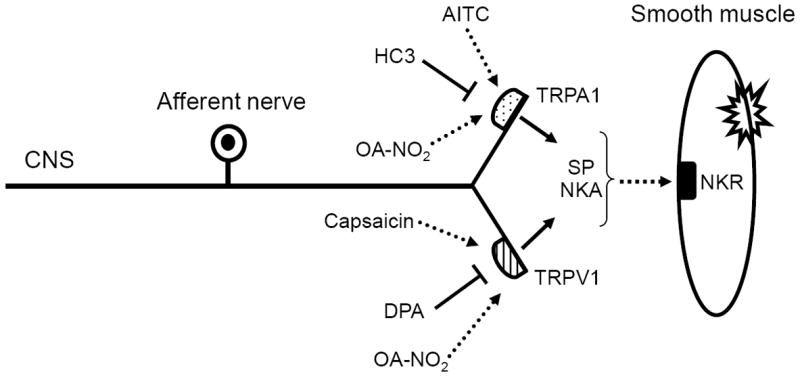
Schematic of OA-NO2 mechanism of action. OA-NO2 activates TRPV1 and TRPA1 channels on capsaicin-sensitive afferent nerve terminals to trigger the release of neurokinins that act on postjunctional receptors to induce smooth muscle contractions. SP – substance P, NKA – neurokinin A. NKR – neurokinin receptor.
Site and mechanism of action of OA-NO2
OA-NO2 increased baseline tone and the amplitude of phasic contractions but had no effect on contraction frequency in rat bladder strips. Pretreatment with CAPS to deplete neurokinin stores and/or desensitize TRPV1-containing nerves significantly reduced the effects of OA-NO2, indicating that one site of action of OA-NO2 is the CAPS-sensitive afferent nerve terminals (Fig. 9). The effects on phasic contraction amplitude and baseline tone were mimicked by TRPV1 and TRPA1 agonists. Furthermore, the effect of OA-NO2 was partially blocked by a TRPV1 antagonist or a TRPA1 antagonist, indicating that the effect is mediated in part by activation of TRPV1 and TRPA1 channels. Ruthenium red, a non-selective TRP channel blocker, was a more effective inhibitor of the effects of OA-NO2 on phasic contraction amplitude, at a concentration (30 uM) that did not affect bladder strip responses to potassium or carbachol. This suggests that additional TRP channels contribute to OA-NO2 actions, although due to potential non-selective effects of ruthenium red, other ion channels could also play a role.
Activation of TRP channels on afferent nerves in the bladder induces the release of neurokinins (Lecci and Maggi, 2001); and agonists for NK1 and NK2 receptors elicit bladder responses similar to those induced by OA-NO2, CAPS or AITC. Also, bladder responses to TRPV1 and TRPA1 agonists (CAPS and AITC, respectively), are reduced by NK receptor antagonists (Saitoh, et al., 2007). Therefore, the effect of NK receptor antagonists to suppress the responses to OA-NO2 supports the conclusion that OA-NO2 activates TRPV1 and TRPA1 channels on afferent nerves in the bladder.
The activation of multiple types of TRP channels in the bladder by OA-NO2 contrasts with the results of a previous study demonstrating a selective action of OA-NO2 on TRPA1 channels on vagal afferent nerves in the lung (Taylor-Clark, et al., 2009). However, there are several differences between these studies that may account for the different results, including different species (mouse versus rat), different neurons (nodose ganglion neurons versus DRG neurons) and the presence of multiple TRP containing cells (e.g. urothelium cells as well as afferent nerves) in the bladder strip preparation. As many TRP channels contain cysteine residues sensitive to Michael addition by electrophilic species, it is not unexpected that multiple TRP channels are targeted by OA-NO2.
Activation of TRP channels by OA-NO2 evokes a depolarization of dissociated DRG neurons in culture (Sculptoreanu, et al., 2010) and induces firing in slowly-conducting vagal afferent nerves (Taylor-Clark, et al., 2009). These findings are consistent with the present experiments showing that pretreatment of bladder strips with TTX significantly reduces, but does not eliminate the effect of OA-NO2 on baseline tone. Alternatively, the effect of OA-NO2 on the amplitude of phasic contractions was not reduced by TTX suggesting that the mechanisms underlying effects on the two types of smooth muscle activity are different. Enhancement of phasic contractions peaked at 15 μm (Fig. 1B), whereas the increase in baseline tone peaked at 33 μm concentration of OA-NO2 (Fig. 1C). This difference in sensitivity to OA-NO2 provides further support for the view that increases in phasic activity and baseline tone might be mediated by different mechanisms.
The TTX-resistant component of the response to OA-NO2 could be related to a subthreshold depolarization of the afferent terminals and influx of Ca2+ without firing that would in turn enhance the spontaneous release of transmitters. Spontaneous release of neurokinins appears to contribute to phasic activity in rat bladder strips because administration of the NK receptor antagonists and TRP channel antagonists reduced basal phasic activity that occured in the absence receptor agonists (Fig. 4). Spontaneous release of transmitters might be triggered by stretch of the bladder strips which activates mechano-sensitive channels in the afferent nerves (de Groat and Yoshimura, 2009). Spontaneous release of acetylcholine from efferent nerves has also been detected in the rat bladder strips (Zagorodnyuk, et al., 2009).
The effects of OA-NO2 also depend on calcium influx through L-type (nifedipine-sensitive) Ca2+ channels, consistent with other studies showing that bladder contractions mediated by activation of NK receptors require activation of L-type Ca2+ channels (Tramontana, et al., 2000). In addition L-type channels might also be involved in NK release from afferent nerves.
Additional sites of action of OA-NO2
OA-NO2 must target receptors in addition to afferent-localized TRP channels in rat bladder strips because NK release from CAPS sensitive neurons can not account for all of the OA-NO2 effect. Furthermore, CAPS pretreatment was highly effective at eliminating the effects of CAPS or AITC in bladder strips, while only partially suppressing the response to OA-NO2. Thus, there may be an OA-NO2 sensitive target localized to the urothelium or to CAPS-insensitive afferent nerves in the muscle layers.
Characteristics of the agonist induced bladder activity
The magnitude and time course of the bladder responses induced by the three agonists were clearly different. Large concentrations of OA-NO2 and AITC elicited approximately equal increases in the amplitude of phasic contractions while a considerably lower concentration of CAPS produced a two fold greater increase in contractions (Fig. 2D), and ~six fold great increase in baseline tone (Fig. 2E). In addition the responses to OA-NO2 and AITC were slower in onset than the response to CAPS reaching a peak after several minutes (Fig. 2 A and C), while the responses to CAPS were more rapid (Fig. 2B). Thus the OA-NO2 evoked responses more closely resembled those evoked by activation of TRPA1 receptors, possibly due to a difference in the kinetics with which the various agonists reached and acted upon the channels. Inward currents evoked by OA-NO2 in DRG neurons were also slower than the currents evoked by CAPS or AITC (Sculptoreanu, et al., 2010).
Inward currents and membrane depolarization evoked by OA-NO2 in dissociated DRG neurons recorded by patch clamp methods occurred at nanomolar concentrations (50-100 nM); whereas the effects in bladder strips required μM concentrations, likely due to challenges presented by the greater diffusional distances and barriers, as well as alternative reactions in the course of diffusion and metabolism (Rudolph 2009). On the other hand CAPS was equally effective at sub- to low-μM concentrations in both preparations.
Putative pathophysiological role of OA-NO2
Because nitro-fatty acids are produced at sites of inflammation in concentrations that are likely to activate TRP channels in afferent nerves they may play a role in pathological conditions in the urinary tract. Activation of TRPV1 in the bladder with CAPS induces painful sensations, neurogenic inflammation including plasma extravasation and bladder overactivity. Activation of TRPA1 also induces bladder overactivity. Thus the initial response to the inflammatory-induced generation of nitro-fatty acids in the bladder might be an enhancement of irritative bladder symptoms. However, a secondary response might be an anti-inflammatory action due to multiple mechanisms including desensitization of TRP channels due to prolonged exposure to the nitrated fatty acids (Sculptoreanu, et al., 2010), suppression of sodium channels and action potential generation in the afferent neurons induced by longer exposures of higher concentrations of OA-NO2 (Sculptoreanu, et al., 2010), upregulation of anti-inflammatory signaling events by OA-NO2 (Khoo 2010) or suppression of NF-κB- regulated cytokine expression (Cui, 2006).
Nitro-fatty acids have been detected in healthy human plasma and urine and are detected at increased levels systemically and in the urine after oral administration of unsaturated fatty acids and nitrite. In the latter instance, the acidic conditions of the gastric compartment induces the formation of the nitrating species nitrous acid (HNO2), which readily induces the nitration of unsaturated fatty acids (Baker, et al., 2005, O’Donnell, et al., 1999, Trostchansky and Rubbo, 2008). Thus, the ultimate permeation of electrophilic nitro-fatty acids from the urine into the bladder wall could activate TRP channels on afferent nerves, interstitial cells or urothelial cells, in turn modulating bladder sensations and voiding function. This raises the possibility that nitro-fatty acids might be useful as anti-inflammatory agents to treat neurogenic and non-neurogenic disorders of the lower urinary tract.
Summary
Our data demonstrate that there are multiple targets of electrophilic nitro-fatty acid reaction in the rat bladder including TRPV1 and TRPA1 channels localized to CAPS-sensitive bladder afferent nerves. OA-NO2 activates these TRP channels, triggering the release of neuropeptides which then act on neurokinin receptors to increase smooth muscle phasic activity. These findings raise the possibility that nitro-fatty acids might be used to modulate bladder function.
Research Highlights.
Nitro-oleic acid increases phasic contractions and baseline tone in rat bladder strips.
Nitro-oleic acid targets TRP channels on capsaicin-sensitive afferent nerves.
Nitro-oleic acid effects involve release of neuropeptides.
The effects require activation of L-type calcium channels.
Nitro-fatty acids can modulate bladder function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int. 2010;106:1114–1127. doi: 10.1111/j.1464-410X.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 2.Artim DE, Kullmann FA, Daugherty SL, Wu HY, de Groat WC. Activation of the nitric oxide-cGMP pathway reduces phasic contractions in neonatal rat bladder strips via protein kinase G. Am J Physiol Renal Physiol. 2009;297:F333–340. doi: 10.1152/ajprenal.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker PR, Schopfer FJ, O’Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free Radic Biol Med. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 7.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Kim DK, Khil LY, Oh U, Kim J, Kwak J. Thimerosal decreases TRPV1 activity by oxidation of extracellular sulfhydryl residues. Neurosci Lett. 2004;369:250–255. doi: 10.1016/j.neulet.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 11.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Pept. 2001;101:1–18. doi: 10.1016/s0167-0115(01)00285-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Jia Z, Soodvilai S, Guan G, Wang MH, Dong Z, Symons JD, Yang T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F942–949. doi: 10.1152/ajprenal.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 15.Maggi CA, Giuliani S, Meli A. Effect of ruthenium red on responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:541–546. doi: 10.1007/BF00260609. [DOI] [PubMed] [Google Scholar]
- 16.Meini S, Patacchini R, Maggi CA. Tachykinin NK1 receptor subtypes in the rat urinary bladder. Br J Pharmacol. 1994;111:739–746. doi: 10.1111/j.1476-5381.1994.tb14800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, rley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitoh C, Kitada C, Uchida W, Chancellor MB, de Groat WC, Yoshimura N. The differential contractile responses to capsaicin and anandamide in muscle strips isolated from the rat urinary bladder. Eur J Pharmacol. 2007;570:182–187. doi: 10.1016/j.ejphar.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sculptoreanu A, Kullmann FA, Artim DE, Bazley FA, Schopfer F, Woodcock S, Freeman BA, de Groat WC. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. J Pharmacol Exp Ther. 2010;333:883–895. doi: 10.1124/jpet.109.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tramontana M, Catalioto RM, Lecci A, Maggi CA. Role of prostanoids in the contraction induced by a tachykinin NK2 receptor agonist in the hamster urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:452–459. doi: 10.1007/s002109900204. [DOI] [PubMed] [Google Scholar]
- 26.Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am J Physiol Renal Physiol. 2010;298:F754–762. doi: 10.1152/ajprenal.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zagorodnyuk VP, Gregory S, Costa M, Brookes SJ, Tramontana M, Giuliani S, Maggi CA. Spontaneous release of acetylcholine from autonomic nerves in the bladder. Br J Pharmacol. 2009;157:607–619. doi: 10.1111/j.1476-5381.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


