Abstract
Periodontitis, the progressive loss of the alveolar bone around the teeth and the major cause of tooth loss in adults, is due to oral microorganisms, including Porphyromonas gingivalis. Periodontitis is associated with a local overly aggressive immune response and a spectrum of systemic effects, but the role of this condition in orodigestive cancers is unclear. We prospectively examined clinically ascertained periodontitis (N = 12 605) and serum IgG immune response to P.gingivalis (N = 7852) in relation to orodigestive cancer mortality among men and women in the National Health and Nutrition Examination Survey III. A detailed oral health exam was conducted from 1988 to 1994 in survey Phases I and II, whereas serum IgG for P.gingivalis was measured from 1991 to 1994 in Phase II only. One hundred and five orodigestive cancer deaths were ascertained through 31 December 2006. Periodontitis (moderate or severe) was associated with increased orodigestive cancer mortality [relative risks (RR) = 2.28, 95% confidence interval (CI) = 1.17–4.45]; mortality risks also increased with increasing severity of periodontal disease (P trend = 0.01). Periodontitis-associated mortality was in excess for colorectal (RR = 3.58; 95% CI = 1.15–11.16) and possibly for pancreatic cancer (RR = 4.56; 95% CI = 0.93–22.29). Greater serum P.gingivalis IgG tended to be associated overall with increased orodigestive cancer mortality (P trend = 0.06); P.gingivalis-associated excess orodigestive mortality was also found for healthy subjects not exhibiting overt periodontal disease (RR = 2.25; 95% CI = 1.23–4.14). Orodigestive cancer mortality is related to periodontitis and to the periodontal pathogen, P.gingivalis, independent of periodontal disease. Porphyromonas gingivalis is a biomarker for microbe-associated risk of death due to orodigestive cancer.
Introduction
The oral periodontium supports the teeth in the alveolar bone of the jaws. Periodontitis, the progressive loss of the alveolar bone around the teeth and the major cause of tooth loss in adults, is due to oral microorganisms, including Porphyromonas gingivalis (1). There is growing evidence that poor periodontal health is associated with systemic health deficits (2,3), including cancer (4,5). Although these disease associations are suspected to have a microbial basis, there is currently no epidemiologic evidence pointing to specific microbial etiologic agents.
In a prospective study of a nationally representative US population sample, we examined whether periodontal disease, assessed by dental exam, is associated with orodigestive cancer mortality. To directly address microbe–cancer associations, we also prospectively investigated the relationship of serum antibody levels for P.gingivalis in relation to orodigestive cancer mortality.
Materials and methods
Study population
The National Health and Nutrition Examination Survey III (NHANES III) survey, which was conducted in two phases between 1988 and 1994 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), was designed to examine the health and nutritional status of the non-institutionalized USA population (6), including estimates for three major racial/ethnic groups: non-Hispanic Whites, non-Hispanic Blacks and Mexican Americans, by oversampling the latter two populations. Data were collected by interview and physical examination in Phases I and II, conducted in Mobile Examinations Centers, including blood sampling in Phase II for measurement of serum P.gingivalis IgG and other serum constituents. All procedures were approved by the NCHS Institutional Review Board, and all subjects provided written informed consent.
For assessment of the relationship of periodontal disease to orodigestive cancer mortality, we restricted eligibility to those who were of age 17 years and older and who had completed the periodontal exam (n = 12 934) in Phase I or Phase II of the survey. Those who had a prior cancer (n = 320), had unknown mortality status (n = 7) or missing smoking status (n = 2) were excluded, resulting in a cohort of 12 605 subjects. For assessment of the relationship of serum P.gingivalis IgG to orodigestive cancer mortality, we restricted eligibility to Phase II participants included above who were also assessed serologically for serum IgG antibody against P.gingivalis (n = 9152). After restricting for age below 17 years (n = 1040), unknown mortality status (n = 7) and prior cancer (n = 290), we included for study 7852 participants in the analysis (exclusion may overlap).
Periodontal disease
For assessment of periodontal disease, periodontal attachment loss and pocket depth were evaluated by dental examiners trained to follow a written set of objective standards to minimize examiner variability by eliminating conditions known to be sources of disagreement (6). If the examiner was equivocal regarding the appropriate score, the lesser score was assigned. All teeth present, excluding roots, were scored. We defined periodontal disease based on the guidelines of the CDC and the American Academy of Periodontology (CDC/AAP) (7). Briefly, subjects were classified as having severe periodontitis if at least two teeth had interproximal (between teeth) attachment loss ≥6 mm and at least one tooth had interproximal pocket depth ≥5 mm. Subjects were classified as having moderate periodontitis if they did not meet criteria for severe periodontitis but they had at least two teeth with interproximal attachment loss ≥4 mm or at least two teeth with interproximal pocket depth ≥5 mm.
Serum IgG antibody to Porphyromonas gingivalis
Serum P.gingivalis IgG was chosen for study to capture evidence of systemic exposure to this bacterium because it is a validated NHANES III periodontal pathogen (8), with well-described periodontal pathogenicity (9). Detailed information on the assay used to measure P.gingivalis IgG has been provided elsewhere (10). Briefly, levels of the serum P.gingivalis IgG were assayed with enzyme-linked immunosorbent assay during the baseline survey or shortly thereafter. Antibody measurements were reported in enzyme-linked immunosorbent assay units of IgG (EU). Based on a previous report in NHANES III validating this assay for periodontal disease, we assigned three categories of P.gingivalis IgG (8,11), associated with none or mild (i.e. normal) (<69 EU), moderate (69.1–119.0 EU) and severe (119.0 + EU) periodontal disease, respectively.
End point ascertainment
Follow-up of the cohort was from the date of the baseline physical and periodontal examination (between 1988 and 1994) until 31 December 2006. Mortality was based on the NHANES III Linked Mortality File (ICD-10; with 113 underlying causes of death), ascertained through probabilistic linkage with the National Death Index (NDI), with cancer deaths coded by the ICD-10 classification. Orodigestive cancers were as follows: lip, oral cavity and pharynx (C00-C14), esophagus (C15), stomach (C16), pancreas (C25), liver (C22) and colon, rectum and anus (C18-C21). Survival follow-up continued until death or 31 December 2006, whichever was earlier.
Statistical analysis
The data were analyzed using SUDAAN (release 9.0.1; Research Triangle Institute) to account for the complex sample design of NHANES III, including the sample weights that were used to account for the unequal probability of selecting individuals and for survey non-response (12). χ2 tests and multiple linear regression were used to assess associations of baseline demographic and health-related characteristics with periodontal disease and serum P.gingivalis IgG. Cox proportional hazards regression analysis was used to compute mortality relative risks (RR) and associated 95% confidence intervals (CI) in relation to categories of periodontal disease and serum P.gingivalis IgG. RR were calculated with time elapsed since survey date (in months) as the time-dependent parameter. RR were adjusted for age and sex and additionally for smoking status (never, former, current and unknown), education, race/ethnicity (non-Hispanic White, non-Hispanic Black and Mexican American and other) and body mass index (BMI) [BMI <25; 25 to <30; 30–39.9 kg/m2; unknown or unlikely (BMI <15 or >40)]. We tested the proportional hazards assumption by modeling interaction terms of time and continuous serum P.gingivalis IgG levels and found no statistically significant deviations. For visual inspection, we also applied non-parametric regression using cubic splines (13) to examine the association between serum P.gingivalis IgG and risk of orodigestive cancer mortality. For this analysis, the number and location of the knots were identified through a stepwise selection process (14). Statistical significance was determined by a two-sided P < 0.05.
Results
Among 12 605 NHANES III participants examined for periodontal disease, 1826 (14.4%) had moderate and 379 (3.0%) had severe periodontal disease. Among 7852 subjects assessed for serum P.gingivalis IgG, levels were significantly greater in study participants with moderate (median: 86.1 EU; inter-quartile range: 70.6–120.6) and severe periodontitis (117.8; 85.3–248.50) compared with normal subjects (70.2; 62.0–88.3). Compared with subjects with (moderate or severe) periodontitis, normal subjects tended to be younger, female, non-Hispanic White, better-educated, never-smokers and of lower BMI (Table I). Participants with low serum P.gingivalis IgG levels (<69.1 EU) also tended to follow these patterns, except that gender differences were not noted and an excess of non-smokers among those with high P.gingivalis titers were noted (Table I).
Table I.
Baseline characteristics of participants according to periodontitis and serum P.gingivalis IgG
| Periodontitis | Serum Porphyromonasgingivalis IgG (EU) | |||||||||
| None | Moderate | Severe | P-valuea | <69.1 | 69.1–119 | >119 | P-valuea | |||
| N | 10400 | 1826 | 379 | N | 2529 | 3681 | 1642 | |||
| Age (years, mean) | 12 605 | 36.2 | 52.2 | 52.0 | <0.0001 | 7852 | 40.4 | 44.1 | 47.1 | <0.0001 |
| Gender (%) | ||||||||||
| Male | 6046 | 47.7 | 62.8 | 74.3 | <0.0001 | 3421 | 47.9 | 48.0 | 50.9 | 0.47 |
| Female | 6559 | 52.3 | 37.2 | 25.7 | 4431 | 52.1 | 52.0 | 49.1 | ||
| Race–ethnicity (%) | ||||||||||
| Non-Hispanic White | 4317 | 74.4 | 67.4 | 59.2 | <0.0001 | 2952 | 87.6 | 68.3 | 50.4 | <0.0001 |
| Non-Hispanic Black | 3695 | 11.0 | 15.8 | 21.5 | 2354 | 5.7 | 12.4 | 22.8 | ||
| Mexican American | 4065 | 6.4 | 6.1 | 4.9 | 2155 | 3.4 | 6.5 | 8.9 | ||
| Other | 528 | 8.2 | 10.8 | 14.4 | 391 | 3.3 | 12.8 | 17.8 | ||
| Education (years, mean) | 12 605 | 13.1 | 12.6 | 10.4 | <0.0001 | 7852 | 13.2 | 12.3 | 12.4 | <0.0001 |
| Smoking status (%) | ||||||||||
| Never | 6892 | 53.7 | 32.2 | 23.0 | <0.0001 | 4275 | 49.8 | 47.0 | 57.1 | <0.0001 |
| Past | 2513 | 20.3 | 30.2 | 29.5 | 1716 | 22.1 | 26.3 | 23.9 | ||
| Current | 3200 | 26.0 | 37.6 | 47.5 | 1861 | 28.1 | 26.7 | 18.9 | ||
| Body mass index (kg/m2) (%) | ||||||||||
| 15.0–24.9 | 5351 | 50.0 | 40.1 | 27.9 | <0.0001 | 3060 | 46.7 | 43.2 | 35.4 | 0.02 |
| 25–29.9 | 4163 | 30.7 | 35.3 | 39.3 | 2675 | 31.9 | 32.8 | 37.3 | ||
| 30.0–39.9 | 2658 | 16.8 | 21.6 | 29.6 | 1829 | 19.0 | 20.8 | 22.7 | ||
| Missing/unreliable | 433 | 2.6 | 3.0 | 3.1 | 288 | 2.4 | 3.2 | 4.5 | ||
Note: all values are weighted.
P-values were based on chi-square test (categorical variables) or one-way analysis of variance (continues variables).
Orodigestive cancer mortality was elevated in subjects with periodontal disease, with adjustment for age and sex (RR = 2.42, 95% CI = 1.48–3.95), and after additional control for smoking, education, race/ethnicity and BMI (RR = 2.28, 95% CI = 1.17–4.45). There was also evidence that risks for orodigestive cancer mortality increased with the severity of periodontal disease (multivariate-adjusted P trend = 0.01) (Table II). Subjects with periodontal disease had excess mortality due to colorectal cancer (deaths = 39), with adjustment for age and sex (RR = 4.34; 95% CI: 1.31–14.44), and after further adjustment for smoking, education, race/ethnicity and BMI (RR = 3.58; 95% CI: 1.15–11.16). Subjects with periodontal disease also tended to have excess risks for pancreas cancer (deaths = 18), with adjustment for age and sex (RR = 4.99; 95% CI: 1.11–22.5) and following additional adjustment for smoking, education, race/ethnicity and BMI (RR = 4.56; 95% CI: 0.93–22.29). No excesses were noted for stomach cancer (deaths = 21), with adjustment for age and gender (RR = 1.02; 95% CI: 0.40–2.55) or with the additional confounders (RR = 0.99; 95% CI: 0.38–2.58), although statistical power was limited in the post hoc analysis (<10%). Other sites of orodigestive cancer had too few deaths for statistical analysis (lip, oral cavity and pharynx deaths = 4; esophagus = 9 and liver = 14) (Supplementary Table 1 is available at Carcinogenesis Online).
Table II.
RRs and 95% CIs for the association between periodontitis and risk of orodigestive cancer mortality
| Periodontitisa | No disease | All periodontitis (moderate and severe combined)a | Moderate periodontitisa | Severe periodontitisa | P trendb |
| Number of orodigestive cancer deaths | 53 | 52 | 41 | 11 | |
| Total sample size | 10 400 | 2205 | 1826 | 379 | |
| Person-time in years | 150 711 | 28 080 | 23 429 | 4651 | |
| Age- and sex-adjusted RR (95% CI) | 1.00 (reference) | 2.42 (1.48–3.95) | 2.21 (1.26–3.89) | 3.80 (1.53–9.44) | 0.0002 |
| Multivariate-adjusted RR (95% CI)c | 1.00 (reference) | 2.28 (1.17–4.45) | 2.22 (1.11–4.46) | 2.64 (0.85–8.23) | 0.01 |
CDC and American Academy of Periodontology definition of periodontal disease: Moderate, two or more teeth with pocket depth ≥5 mm or two or more teeth with attachment loss ≥4 mm; Severe, one or more teeth with pocket depth ≥5 mm and two or more teeth with attachment loss ≥6 mm.
P trend was tested for no disease, moderate periodontitis and severe periodontitis.
Multivariate model adjusted for age, sex, smoking status, education, race/ethnicity and BMI.
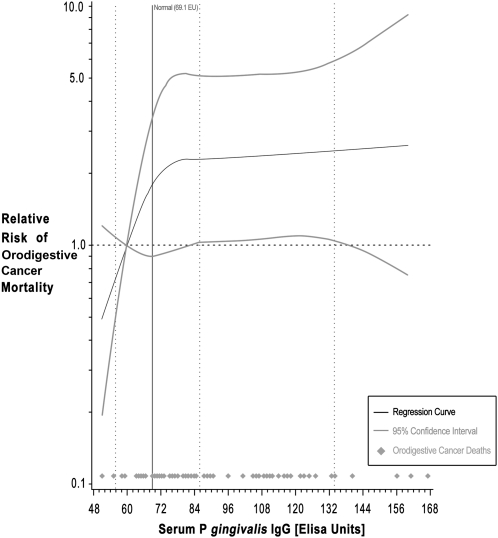
Participants with high levels of serum antibody to P.gingivalis (≥69.1 EU) also tended to have excess orodigestive cancer mortality overall (RR = 3.03; 95% CI: 0.99–9.31) and after exclusion of subjects with clinically apparent periodontal disease (RR = 2.25, 95% CI = 1.23–4.14) (Table III). Non-parametric trend analysis for all subjects (Figure 1) showed increasing orodigestive cancer mortality with increasing P.gingivalis IgG levels, although the dose-response was not linear, tending to flatten at higher IgG levels.
Fig. 1.
Non-parametric regression curve for the association between serum Porphyromonas gingivalis IgG and orodigestive cancer mortality. The multivariate model (with four knots) is adjusted for age, sex, smoking status, education, race/ethnicity and BMI.
Table III.
RRs and 95% CIs for the association between serum P.gingivalis IgG and risk of orodigestive cancer mortality
| Serum P.gingivalis IgG | <69.1 EU | 69.1–119.0 EU | >119.0 EU | P trend | 69.1 + EU |
| Number of orodigestive cancer deaths | 13 | 48 | 26 | 74 | |
| Total sample size | 2529 | 3681 | 1642 | 5323 | |
| Person-time in years | 31 552 | 45 075 | 20 128 | 65 203 | |
| Age- and sex-adjusted RR (95% CI) | 1.00 (reference) | 3.25 (1.04–10.13) | 2.45 (0.60–10.06) | 0.07 | 3.03 (0.94–9.79) |
| Multivariate-adjust ed RR (95% CI)a | 1.00 (reference) | 3.15 (1.06–9.34) | 2.61 (0.64–10.59) | 0.06 | 3.03 (0.99–9.31) |
Multivariate model adjusted for age, sex, smoking status, education, race/ethnicity and BMI.
Discussion
Our investigation in NHANES III, a nationally representative population-based cohort study, showed increasing risk for orodigestive cancer mortality in relation to increasing severity of periodontal disease and in relation to serum P.gingivalis IgG, a biomarker for exposure to this periodontitis-related pathogen. The observed associations remained statistically significant following control for age, sex, smoking, education, race/ethnicity and BMI. Although numbers were small for cancer site-specific analyses, clinically defined periodontitis tended to be associated with colorectal and pancreas cancer in our study.
Several other studies have related cancer risks to periodontal disease and tooth loss, a generally accepted surrogate marker in adults for periodontitis (1). In NHANES I, non-significant age- and gender-adjusted excesses of pancreas cancer were found in subjects with periodontitis; excesses for colon and stomach cancer were not noted (15). In a hospital-based case–control study of 14 common cancer types in Japan (16), self-reported tooth loss was associated with head and neck (borderline significant) and esophagus cancer. In the Health Professional Follow-up Study cohort (17,18), self-reported periodontitis was associated with increased risk for all cancer and for pancreas cancer, whereas tooth loss was associated with a borderline significant association with all cancer. Greater tooth loss was associated with excesses of esophageal squamous cell carcinoma and gastric cardia and non-cardia adenocarcinoma, in studies in China (19) and Finland (20), whereas excesses of pancreas cancer were also reported from the Finnish study (21). Recent reviews indicate that there is growing evidence of oral health–orodigestive cancer risk associations, with the strongest evidence for associations with pancreas cancer (4,5). Farrell et al. (22) recently reported that oral microbiome profiles significantly differ in pancreatic cancer patients and controls.
Although several studies are pointing to associations of poor oral health with increased risk for cancer and other diseases (2–5), our study is the first to relate a specific oral microbe, P.gingivalis, to orodigestive cancer. Furthermore, our observation that P.gingivalis is associated with risk in subjects not exhibiting overt periodontal disease is a strong indicator that microbial factors play a role in orodigestive carcinogenesis independent of their association with periodontal disease. In addition to their role in periodontal disease and tooth loss, oral bacteria colonize the gut and enter the blood after mastication, personal oral hygiene and dental procedures (23–27) and provide source of ligands for toll-like receptors (28), membrane receptors involved in innate immune regulation by recognition of pathogen-associated molecular patterns from a wide spectrum of infectious agents. It is also possible that inflammation due to immunologic response to oral bacteria and their toxins (3,29) may play an important role in oral and gastrointestinal carcinogenesis (30,31). Alternatively, oral bacteria may be associated with cancer risk by local metabolism of activating carcinogens from alcohol and smoking—risk factors for several orodigestive cancers—and systemic release of the activated carcinogens, which has been most clearly demonstrated for alcoholic beverage-associated ethanol metabolism by oral microbes (32). Finally, oral bacterial profiles could be a marker of an individual’s unmeasured proinflammatory susceptibility related to inherent genetic and immune system deficiencies.
Our study was limited in that it was based on mortality rather than incidence and results were derived from small numbers of site-specific orodigestive cancer deaths, thus we had limited power for evaluation of mortality risks for specific cancers. Although P.gingivalis is a well-characterized oral bacteria, serum antibody assessments are indirect measures for orodigestive bacterial exposure, based on individually variable vascular exposure and immunologic response. Our study points to P.gingivalis as a potential etiologic agent, yet we recognize that this association may be serving as a proxy biomarker for other pathogenic agents and that definitive conclusions will require direct microbiome-wide evaluations of oral microbes, as are recently coming into epidemiologic use (33). Strengths of this study include the prospective design in a nationally representative population, including ethnic minority groups, a detailed periodontal exam characterizing severity of periodontitis and the availability of pre-diagnostic serum P.gingivalis IgG levels as a periodontal pathogen biomarker. With these resources, we were able to evaluate dose-response relationships using clinic and biomarker-based risk factor measures.
In summary, we found that periodontal disease was associated with orodigestive cancer mortality and identified P.gingivalis as a specific and potentially independent microbial marker for risk of orodigestive cancer death. These findings may reinforce public health recommendations for the maintenance of good oral health as a means of orodigestive cancer prevention and point to the need for microbiome-based approaches to identify the potentially broad spectrum of microbial drivers of orodigestive cancer mortality.
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
This study was supported by R01 CA159036 from National Cancer Institute and Career Development Award by American Association for Cancer Research (AACR).
Supplementary Material
Acknowledgments
We thank Mr. John Zurik for graphic support.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- EU
enzyme-linked immunosorbent assay unit
- NHANES
National Health and Nutrition Examination Survey
- RR
relative risks
References
- 1.Pihlstrom BL, et al. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Lamster IB, et al. The relationship of periodontal disease to diseases and disorders at distant sites: communication to health care professionals and patients. J. Am. Dent. Assoc. 2008;139:1389–1397. doi: 10.14219/jada.archive.2008.0051. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo G, et al. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur. J. Intern. Med. 2010;21:496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Meyer MS, et al. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick SG, et al. The association between periodontal disease and cancer: a review of the literature. J. Dent. 2010;38:83–95. doi: 10.1016/j.jdent.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Centers for disease control and prevention. The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) Reference Manuals and Reports. Bethesda, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 7.Page RC, et al. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 8.Dye BA, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J. Periodontol. 2009;80:634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 9.Offenbacher S, et al. Periodontal disease at the biofilm-gingival interface. J. Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 10. National Health and Nutrition Examination Survey Codebook for Data Production (1991–1994) (NHANES III) Periodontal Pathogens: Serum Actinobacillus actinomycetemcomitans Antibody and Porphyromonas gingivalis Antibodies http://www.cdc.gov/nchs/data/nhanes/nhanes3/depp.pdf.
- 11.Noble JM, et al. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry. 2009;80:1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn EL, et al. Analysis of Health Surveys. New York: Wiley; 1999. [Google Scholar]
- 13.Durrleman S, et al. Flexible regression models with cubic splines. Stat. Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 14.Govindarajulu U, et al. Comparing smoothing techniques for modeling exposure-response curves in Cox models. Stat. Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 15.Hujoel PP, et al. An exploration of the periodontitis-cancer association. Ann. Epidemiol. 2003;13:312–316. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 16.Hiraki A, et al. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol. Biomarkers Prev. 2008;17:1222–1227. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J. Natl Cancer Inst. 2007;99:171–175. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 18.Michaud DS, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abnet CC, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. (Translated from Eng) Cancer Causes Control. 2001;12:847–854. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 20.Abnet CC, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand. J. Gastroenterol. 2005;40:681–687. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 21.Stolzenberg-Solomon RZ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am. J. Clin. Nutr. 2003;78:176–181. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- 22.Farrell JJ, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2011;61:582–588. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai T. Periodontal bacteremia and various vascular diseases. J. Periodontal. Res. 2009;44:689–694. doi: 10.1111/j.1600-0765.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 24.Crasta K, et al. Bacteraemia due to dental flossing. J. Clin. Periodontol. 2009;36:323–332. doi: 10.1111/j.1600-051X.2008.01372.x. [DOI] [PubMed] [Google Scholar]
- 25.Lockhart PB, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahrani-Mougeot FK, et al. Diverse and novel oral bacterial species in blood following dental procedures. J. Clin. Microbiol. 2008;46:2129–2132. doi: 10.1128/JCM.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen I, et al. Update on bacteraemia related to dental procedures. Transfus. Apher. Sci. 2008;39:173–178. doi: 10.1016/j.transci.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Chinen T, et al. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med. 2010;207:2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meurman J. Oral microbiota and cancer. J. Oral Microbiol. 2010;2:1–10. doi: 10.3402/jom.v2i0.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrow B, et al. Inflammation and the development of pancreatic cancer. Surg. Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 31.Greer JB, et al. Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Salaspuro M. Acetaldehyde: a cumulative carcinogen in humans. Addiction. 2009;104:551–553. doi: 10.1111/j.1360-0443.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J, et al. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.