Abstract
Plants are known to mount a defensive response when exposed to volatile chemicals from other plants, but the critical concentration required for this response is not known. We showed that intermittent exposure over a period of 3 weeks to trace amounts (less than 140 pptV) of green leaf volatiles emitted by a freshly damaged Arabidopsis plant induced physiological (defensive) responses in undamaged neighbouring plants. These results demonstrated that plants can respond to long-term repeated exposures to subcritical amounts of chemical signals.
An increasing number of laboratory and field studies have demonstrated that plants can respond defensively to green leaf volatiles (GLVs) emitted from nearby plants (both con- and hetero-specific) that have been damaged by herbivores1,2,3. A crucial question that remains to be answered is how far these plant–plant signals can travel and, given the dilution of airborne chemicals over distance, how sensitive the recipient plant is to the concentration of volatiles.
Some synthetic GLVs, such as (Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexenol, induce gene expression in undamaged Arabidopsis thaliana plants4,5. Some of the induced genes are involved in the phytooxylipin pathway, through which GLVs are produced4,5. Thus, a genetic mechanism of plant–plant signalling in Arabidopsis involves the activation of the phytooxylipin pathway in receiver plants in response to GLVs from neighbouring, damaged plants. Here, we ask how many molecules of GLVs from a damaged Arabidopsis plant are needed to trigger the phytooxylipin pathway in a neighbouring undamaged plant.
Caterpillars of the cabbage white butterfly (Pieris rapae) are major herbivores of plants in the family Brassicaceae and they are, in turn, parasitized by females of the wasp, Cotesia glomerata. These wasps can significantly reduce P. rapae populations6 and are attracted by GLVs that are released when plants are mechanically damaged7. In Arabidopsis, (E)-2-hexenal and (Z)-3-hexenyl acetate have been shown to recruit C. glomerata7. If the phytooxylipin pathway in undamaged Arabidopsis is indeed activated by GLVs from wounded neighbours, the undamaged plants would be able to emit larger amounts of GLVs should they be attacked by caterpillars and would thus be more attractive to C. glomerata than otherwise. To test this possibility, we used Arabidopsis ecotype Ler-0 wild type (wt) and hpl1 mutants, which produce only trace amounts of GLVs.
In most studies on plant–plant signalling under laboratory conditions, undamaged plants received volatiles that were produced continuously by emitter plants, such as herbivore-damaged plants1,2. However, in the field, continuous exposure is unlikely because of fluctuating wind directions and air turbulence. Therefore, in this study, the emitter's volatiles were released intermittently over 3 weeks.
Results
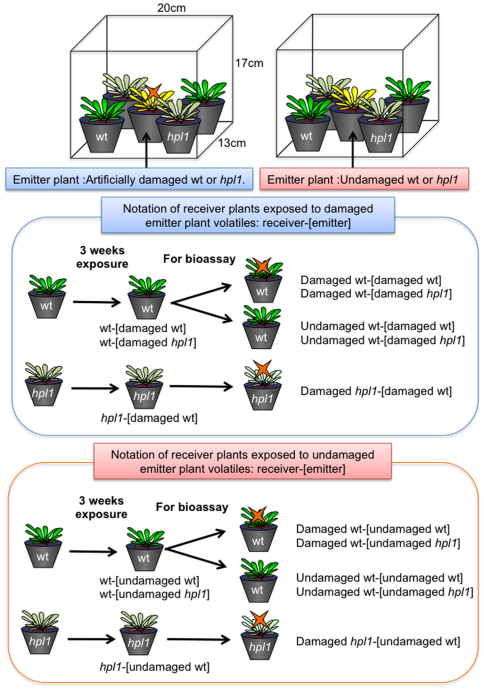
Plant preparation and the experimental design are detailed in the Methods section. Each pot contained five plants of the same genotype. Two pots of wt plants and two pots of hpl1 plants were grown equidistant from one pot of emitter plants (either wt or hpl1: three-weeks-old) in an open plastic container (20×17×13 cm) in a climate-controlled room (Figure 1). Within each container, there was no artificially generated airflow. Emitter plants were injured twice per week for a period of three weeks. In this way, the receivers were intermittently exposed to volatiles from Arabidopsis plants with artificially damaged leaves. The different treatments (denoted in brief as receiver-[emitter]; see Figure 1 and Methods section) were: (I) wt receiver plants exposed to volatiles from damaged wt emitters: wt-[damaged wt], (II) hpl1 receivers exposed to volatiles from damaged wt emitters: hpl1-[damaged wt], (III) wt receivers exposed to volatiles from damaged hpl1 plants: wt-[damaged hpl1] (Figure 1). In the “control” condition (IV), the emitter plant was not damaged: wt-[undamaged wt], hpl1-[undamaged wt], and wt-[undamaged hpl1], respectively (Figure 1).
Figure 1. The experimental set-up is shown in the top part.
Receiver plants [either wt (plants with green leaves) or hpl1 (plants with pale green leaves)] were exposed to volatiles from emitter plants located at the center of the box. The emitter plants (plants with yellow leaves) were either artificially damaged with scissors or undamaged. The emitter plants were damaged twice a week for three weeks. After 3 weeks exposure, the receiver plants were subjected to choice bioassays with C. glomerata females. The middle part shows the notation of receiver plants exposed to damaged emitter plant voaltiles. Notation is shown as emitter-[receiver]. For the bioassay, the exposed plants were either artificially damaged with scissors or undamaged. The bottom part shows the notation of receiver plants exposed to undamaged emitter plant voaltiles.
After the exposure period, receiver plants were used for two-choice bioassays with C. glomerata females. Two receiver pots were positioned in a plastic cage (25×35×30 cm) in a climate-controlled room. Individual wasps were released from positions equidistant from the two pots. Wasps were considered to have chosen a plant when they landed and initiated ambulatory search for their hosts, i.e. the caterpillars, on the leaves; next, they were removed with an aspirator. Plants were either undamaged or artificially damaged, and the bioassays were conducted immediately after the plants were injured.
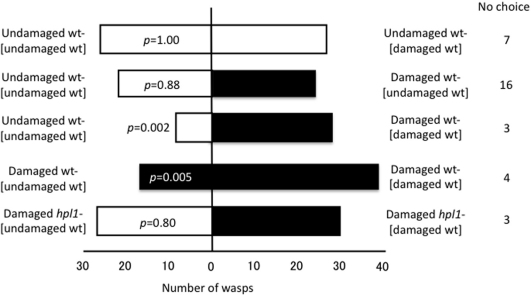
Cotesia glomerata showed no statistically significant preference between undamaged wt-[undamaged wt] and undamaged wt-[damaged wt] (p = 1.00), indicating that prior exposure to wt emitters was not sufficient to induce GLV production in receiver plants. When offered a choice between undamaged and damaged wt-[undamaged wt], wasps again showed no significant preference (p = 0.88) (Figure 2) presumably because GLV production had not yet noticeably increased in response to the damage. In contrast, C. glomerata showed a significant preference for damaged wt-[damaged wt] over undamaged wt-[undamaged wt] (p = 0.002) and for damaged wt-[damaged wt] over damaged wt-[undamaged wt] (p = 0.005) (Figure 2). C. glomerata did not show a significant preference for damaged hpl1-[damaged wt] over damaged hpl1-[undamaged wt] (p = 0.80) (Figure 2), indicating that C. glomerata responded to the slight increase of GLVs emitted from damaged wt-[damaged wt] over damaged wt-[undamaged wt]. Thus, damaged Arabidopsis plants become more attractive to C. glomerata after repeated exposure to trace amounts of GLVs from damaged wt, which represents evidence of plant–plant signalling.
Figure 2. Flight response of C. glomerata to Arabidopsis wt plants that had been previously exposed to wt volatiles under different conditions.
Undamaged wt-[damaged wt]: undamaged wt receiver plants exposed to volatiles from damaged wt emitters; Undamaged wt-[undamaged wt]: undamaged wt receiver plants exposed to volatiles from undamaged wt emitters; Damaged wt-[damaged wt]: damaged wt receiver plants exposed to volatiles from damaged wt emitters; Damaged [undamaged wt]: damaged wt receiver plants exposed to volatiles from undamaged wt emitters; Damaged hpl1-[damaged wt]: damaged hpl1 receiver plants exposed to volatiles from damaged wt emitters; Damaged hpl1-[undamaged wt]: damaged hpl1 receiver plants exposed to volatiles from undamaged wt emitters. Choice data were analysed with a binomial test (α = 0.05).
We also conducted bioassays on receiver plants that had been exposed to emitters for a shorter duration (two exposures over one week), but C. glomerata showed no preferences between damaged wt-[damaged wt] and damaged wt-[undamaged wt]: 12 wasps landed on damaged wt-[damaged wt], 18 wasps on damaged wt-[undamaged wt], and 10 wasps made no choice (p = 0.36), although the wasps showed a significant preference in the same experiment when it lasted 3 weeks. Thus, intermittent exposure to damaged plants must occur for periods longer than one week to elicit a defence response in receiver plants.
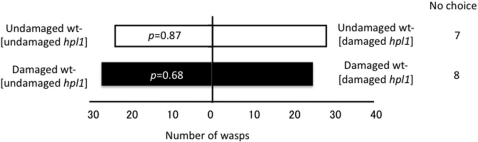
We then conducted bioassays with plants exposed to hpl1 emitters, to exclude the possibility that volatiles other than those produced by the phytooxylipin pathway elicited responses in the wasps. Wasps were offered choices between undamaged wt-[undamaged hpl1] and undamaged wt-[damaged hpl1], and between damaged wt-[undamaged hpl1] and damaged wt-[damaged hpl1]. They showed no significant preferences in either of these trials (p = 0.87 and 0.68, respectively) (Figure 3). These data indicate that the hpl1 genotype, with suppressed GLV production, did not produce sufficient volatiles via other pathways for plant–plant defensive signalling in Arabidopsis.
Figure 3. Flight response of C. glomerata to Arabidopsis wt plants that had been previously exposed to hpl1 volatiles under different conditions.
Undamaged wt-[damaged hpl1]: undamaged wt receiver plants exposed to volatiles from damaged hpl1 emitters; Undamaged wt-[undamaged hpl1]: undamaged wt receiver plants exposed to volatiles from undamaged hpl1 emitters; Damaged wt-[damaged hpl1]: damaged wt receiver plants exposed to volatiles from damaged hpl1 emitters; Damaged wt-[undamaged hpl1]: undamaged wt receiver plants exposed to volatiles from undamaged hpl1 emitters. Choice data were analysed with a binomial test (α = 0.05).
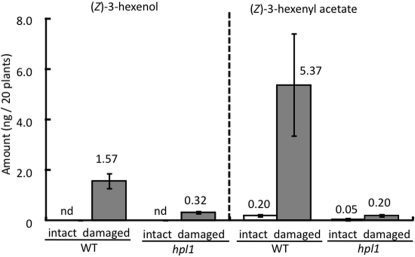
Finally, we analysed the volatiles produced by twenty damaged plants (four injuries per plant) of both wt and hpl1 to show that these genotypes differ only in the relative amounts of GLVs released. Only two GLVs, (Z)-3-hexenol and (Z)-3-hexenyl acetate, were detected in the headspace of either wt or hpl1, and both compounds showed characteristic increases in concentration in response to artificial damage in both genotypes (Figure 4). Based on these data, we estimated that the five damaged wt plants (one injury per plant) used in our experiments released a total of 98.0 pg (7.0 parts per trillion by volume, pptV) of (Z)-3-hexenol and 335.5 pg (17.0 pptV) of (Z)-3-hexenyl acetate, or a total of ∼24 pptV of GLVs. Similarly, we estimated that the five damaged hpl1 plants (four injuries per plant) emitted a total of 20.0 pg (1.4 pptV) of (Z)-3-hexenol and 12.5 pg (0.6 pptV) of (Z)-3-hexenyl acetate, or a total of ∼2.0 pptV GLVs, in our experiments. These data suggest that repeated exposure to ∼2.0 pptV GLVs was not sufficient, whereas ∼24 pptV GLVs was sufficient to induce plant–plant signalling in Arabidopsis. Because the absorbent used in the chemical analyses collects most but not all volatiles in the headspace, the actual GLV threshold is likely to be somewhat higher.
Figure 4. Volatiles in the headspace of artificially damaged wt and hpl1 Arabidopsis plants.
Four leaves per plant were damaged (one 5-mm slit per leaf). Twenty plants were prepared for both wt and hpl1. Only (Z)-3-hexenol and (Z)-3-hexenyl acetate were detected in the headspace of either plant genotype.
To check these measurements, we also quantified GLV production by fully disrupted Arabidopsis leaf tissue. Wt produced 496 nmol GLVs per g fresh weight. We estimated that a single artificial wound was 0.01 mm wide × 5 mm long, corresponding to ∼7.5 μg of leaf tissue. We calculated such an injury produced ∼3.7 pmol of GLVs. Our emitter experiments involved five leaf-cut injuries (one per plant), so the expected concentration of GLVs in the exposure trials should have been 18.5 pmol in a 3-L volume, or ∼140 pptV for wt plants. Thus, we concluded that a threshold occurs between ∼24 pptV of GLVs (based on volatiles from whole but injured plants) and ∼140 pptV of GLVs (based on volatiles from fully disrupted leaves) per exposure. Levels of GLV exposure above this threshold were sufficient for plant–plant signalling in Arabidopsis (i.e., to prime the biochemical pathway that produced the GLVs attracting the wasp C. glomerata).
Discussion
In this study, we focused on the ecological context of plant–plant signalling by assessing the olfactory responses of C. glomerata, a parasitoid of a ravenous herbivore. We showed that GLVs were involved in plant–plant signalling in Arabidopsis (ecotype Ler-0) and that the amounts needed to elicit a molecular / physiological response in receiver plants was in the range of 24–140 pptV per exposure for a total of six exposures over three weeks. After only two exposures in a single week, there was no evidence of plant–plant signalling, however. These observations indicate that repeated exposure accumulates into a detectable response with a threshold between two and six GLV exposures at this concentration. One might suspect that the wasps responded to HIPV that originated from the emitters and had only been adsorbed and re-released by the receivers8,9. However, this possibility is unlikely because wasps showed equal distributions between undamaged wt-wt control and undamaged wt-wt, the latter of which would have absorbed and re-released the volatiles had that been the case. Molecular biological and genomic approaches may help to determine why multiple exposures over three weeks were required for plant-plant signalling in Arabidopsis. Arabidopsis is an ideal model system for such studies because its physiology is well studied and because mutants of many important biochemical pathways are available.
The ecological relevance of plant-plant signalling has been questioned based on the assumption that volatiles will be diluted to extremely low concentrations under natural conditions of fluctuating wind direction and air turbulence10. However, this objection is rarely raised in discussions of olfactory signalling in animals. Behavioural responses of mosquitoes (Aedes aegypti) to ammonia presented together with lactic acid were elicited at concentrations ranging from 17 ppbV to 17 ppmV11. Salazar and Laska12 reported on the olfactory sensitivity to aliphatic esters in a spider monkey, and they found that, in several cases, individual monkeys could discriminate concentrations below 1 ppbV. Although their experimental conditions differed from ours, our results showed that plant–plant signalling may be just as acute as olfactory signalling in animals. This suggests that plant-plant signalling in Arabidopsis could occur under natural conditions.
Methods
Introgression of the hpl mutant gene into Arabidopsis thaliana Ler
The hpl mutant gene in Col-0 was introgressed into Ler through backcrossing and subsequent selection for four generations. At each F2 generation, hydroperoxide lyase activity was determined as previously described13, and the progeny showing the hpl phenotype were chosen for the next round of backcrossing. Genotyping was performed on the fourth generation progeny using representative chromosomal markers. Progeny having Col-0 markers near the HPL locus on chromosome 4 and the Ler genotype at all other markers were chosen for our experiments. Supplementary Figure 1 shows the genetic map of the hpl1 mutants used in this study.
Experimental design
The bioassay experiments were conducted in a climate-controlled room at 25 ± 2°C, 50–70% relative humidity, and a 16-h light/8-h dark cycle. Five Arabidopsis plants were grown in soil (1:1 mixture of expanded vermiculite and perlite) in a pot (5 cm diameter × 7 cm high) for three weeks before the experiments began. We watered the plants every other day (Hyponex diluted 1000×; Hyponex Japan Co., Ltd., Osaka, Japan). For the volatile exposure, a single pot (either wt or hpl1) was placed in the centre of a small container (20 cm wide, 13 cm long × 17 cm high); these plants served as the volatile emitters. Two wt and two hpl1 pots were placed in an alternating arrangement in the corners of the container to serve as receiver plants. There was no airflow in the container. Each emitter-receiver combination was replicated four to five times on different experimental days.
In all cases, damage was induced by cutting one 5-mm long slit in a leaf using ophthalmologic surgical scissors. When the emitter was wt, one leaf per plant was damaged. When the emitter was hpl1, four leaves per plant were damaged, because the production of GLVs was expected to be lower than in wt plants. Emitter plants were damaged twice per week (at 10 AM on Monday and Friday) for three consecutive weeks. The different treatments are denoted as follows: wt-[damaged wt] were wt receiver plants exposed to volatiles from damaged wt emitters; wt-[damaged hpl1] were wt receiver plants exposed to volatiles from damaged hpl1 plants; hpl1-[damaged wt] were hpl1 receiver plants exposed to volatiles from damaged wt emitters; wt-[undamaged wt] were wt receiver plants exposed to volatiles from undamaged wt emitters; wt-[undamaged hpl1] were wt receiver plants exposed to volatiles from undamaged hpl1 plants; and hpl1-[undamaged wt] were hpl1 receiver plants exposed to volatiles from undamaged wt emitters (Figure 1).
After the three-week exposure period, receiver plants were used for choice bioassays with C. glomerata females. Individual wasps were released from a glass tube positioned halfway between two receiver pots in a cage (25 cm wide × 35 cm long × 30 cm high) with three windows covered by nylon gauze and a door to introduce plants and wasps. The cage was placed in the climate-controlled room described above. The wasps were considered to have chosen a plant when they landed and initiated an ambulatory search for prey; they were then removed with an aspirator. If a wasp did not land on either of the two pots within 30 min, it was scored as a no-choice result. The receiver plants were either undamaged or artificially injured as described above; damaged receivers were used in the bioassays immediately after the injury. One leaf per plant was damaged for wt plants; four leaves per plant were damaged for hpl1 plants. Five to ten female wasps were used in each trial. The trial was repeated four to five times for each emitter-receiver combination on different experimental days.
Statistical analysis
Choice tests were statistically analysed using a binomial test under the null hypothesis that wasps had a 1:1 distribution over the two groups of plants. The critical value for significance was α = 0.05.
Chemical analysis of volatiles
We injured 4–5-week-old Arabidopsis (both wt and hpl1) by making one 5-mm slit (as described above) on each of four leaves per plant. Then the plants were cut just above the ground. Twenty damaged Arabidopsis were placed in in a 35-ml glass bottle with moist cotton wool. We collected organic volatiles by adsorbing them onto four Twisters [polydimethylsiloxane (PDMS)-coated stir bar, film thickness 0.5 mm, 10-mm length; Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany] hanging stationary in the glass bottle for two hours (25°C). We collected volatiles from undamaged Arabidopsis using the same procedure. The collected volatiles were analysed by GC-MS with an HP-5MS capillary column (Agilent Technologies) equipped with a thermo-desorption system, a cooled injection system and a cold trap system (Gerstel GmbH & Co. KG). The oven temperature of the GC was programmed to rise from 40°C (9-min hold) to 280°C at 10°C/min. The compounds were identified by comparing the GC retention times and mass spectra with those of known reference compounds, including synthetic (Z)-3-hexenol and (Z)-3-hexenyl acetate. Reference compounds were used to quantify (Z)-3-hexenol and (Z)-3-hexenyl acetate in the headspace. Different concentrations of these known compounds were vaporized into identical glass bottles, and headspace collection and chemical analyses were done as described above. We used the peak area of these known concentrations to make a calibration curve for each compound and we used this curve for quantification.
We also measured GLVs produced by fully disrupted wt and hpl1 leaves as follows. The aboveground parts of Arabidopsis plants with fully developed leaves (45 days old) were cut off with a razor blade, weighed and placed into a glass vial (22 ml, Perkin Elmer, Waltham, MA, USA) with 18 stainless steel beads (3 mm i.d.). When the volatiles in undamaged tissues were analysed, 2 ml of saturated CaCl2 solution was added to kill any enzymatic activity. The vial was sealed tightly with a butyl stopper and crimp top seal (National Scientific, Rockwood, TN, USA), then the tissues were completely disrupted by vortexing the vial vigorously. When volatiles formed in completely disrupted leaves were analysed, the tissues were disrupted without saturated CaCl2 solution and incubated for 5 min at 25°C. After 5 min, 2 ml of saturated CaCl2 solution was added to halt any enzyme reactions.
SPME fibre (50/30 μm DVB/Carboxen/PDMS, Supelco, Bellefonte, PA, USA) was exposed to the headspace of the vial for 30 min at 25°C. The fibre was inserted into the insertion port of the GC-MS (QP-5050, Shimadzu, Kyoto, Japan) equipped with 0.25 μm × 30 m Stabiliwax column (Restek, Bellefonte, PA, USA). The column temperature was programmed to hold at 40°C for 1 min, then to rise at a rate of 15°C min−1 to a final hold-temperature of 180°C for 1 min, with a carrier gas (He) at 1 min−1. The glass insert was an SPME Sleeve (Supelco). Splitless injection with a sampling time of 2 min was used. The fibre was kept inserted into the injection port for 10 min to bake out any compounds from the matrix. The temperature of the injector and interface was 200 and 230°C, respectively. The mass detector was operated in an electron impact mode with ionization energy of 70 eV. To assign each compound, retention indices and MS profiles of corresponding authentic compounds were used. For quantification, aqueous solutions of each compound (suspended in the presence of a small amount of Tween 20) were mixed together in different ratios and then mixed with 1 ml of a saturated CaCl2 solution in a glass vial. The volatiles were analysed with SPME-GC-MS as described above, and a calibration curve for each compound was constructed. (Z)-3-hesenal was a generous gift from Zeon Co (Tokyo, Japan).
The total amount of all three GLVs ((Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexenol) produced by fully disrupted tissue was 496 nmol GLV per g fresh weight (Supplementary Figure 2). This value was assumed to be the greatest amount of GLVs the leaves could potentially synthesize. An average wt leaf weighed 150 μg/mm2, and we estimated that a single injury measured 0.01 mm × 5 mm, or 0.05 mm2 and 7.5 µg of leaf tissue. Thus, if an entire leaf produced 496 nmol/g, we estimated that a single wound would produce 3.7 pmol GLVs. Our experiments on wt plants involved five injuries (one injury per plant × five plants), so the expected concentration of GLVs in the exposure trials should have been 18.5 pmol in a 3-L volume, or ∼140 pptV for wt plants.
Author Contributions
KS designed and conducted the bioassay. RO conducted the chemical analyses. KM isolated mutants and conducted molecular biological analyses. MS and JT designed and supervised the entire study.
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Dr. Robert Schuurink for comments on the manuscript, and Dr Leah Larkin for editorial support. This research was supported in part by a Grant-in-Aid for Scientific Research (S) (No. 19101009) from the Bio-oriented Technology Research Advancement Institution, JSPS Core-to-Core Project, and by the Global Center of Excellence Program “Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem” of Kyoto University, Japan.
References
- Heil M. & Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 (2010). [DOI] [PubMed] [Google Scholar]
- Arimura G-I., Matsui K. & Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: Proximate factors and their ultimate functions. Plant Cell Physiol. 50, 911–923 (2009). [DOI] [PubMed] [Google Scholar]
- Matsui K., Kurishita S., Hisamitsu A. & Kajiwara T. A lipid-hydrolysing activity involved in hexenal formation. Biochem. Soc. Trans. 28, 857–860 (2000). [PubMed] [Google Scholar]
- Bate N. J. & Rothstein S. J. C-6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 16, 561–569 (1998). [DOI] [PubMed] [Google Scholar]
- Kishimoto K., Matsui K., Ozawa R. & Takabayashi J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 46, 1093–1102 (2005). [DOI] [PubMed] [Google Scholar]
- Ohsaki N. & Sato Y. Avoidance mechanisms of 3 Pieris butterfly species against the parasitoid wasp Apanteles glomeratus. Ecol. Entomol. 15, 169–176 (1990). [Google Scholar]
- Shiojiri K., et al.. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 32, 969–979 (2006). [DOI] [PubMed] [Google Scholar]
- Choh Y. et al.. Lima bean leaves exposed to volatiles from herbivore-induced conspecific plants emit carnivore attractants: active or passive response? J. Chem. Ecol. 30, 1305–1317 (2004). [DOI] [PubMed] [Google Scholar]
- Himanen S. J. et al.. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants – a mechanism for associational herbivore resistance? New Phytol. 186, 722–732 (2010). [DOI] [PubMed] [Google Scholar]
- Preston C. A., Laue G. & Baldwin I. T. Plant-plant signalling: Application of trans- or cis-methyl jasmonate equivalent to sagebrush releases does not elicit direct defenses in native tobacco. J. Chem. Ecol. 30, 2193–2214 (2001). [DOI] [PubMed] [Google Scholar]
- Geier M., Bosch O. J. & Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem. Senses 24, 647–653 (1999). [DOI] [PubMed] [Google Scholar]
- Salazar L. T. H., Laska M. & Luna E. R. Olfactory sensitivity for aliphatic esters in spider monkeys (Ateles geoffroyi). Behav. Neurosci. 117, 1142–1149 (2003). [DOI] [PubMed] [Google Scholar]
- Duan H., Huang M. Y., Palacio K. & Schuler M. A. Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signalling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol. 139, 1529–1544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures