Abstract
As part of our program to develop breast cancer specific therapeutic agents we have synthesized a conjugate-agent that is a conjugate of the steroidal anti-estrogen and the potent cytotoxin doxorubicin. In this effort we employed a modular assembly approach to prepare a novel 11β-substituted steroidal anti-estrogen functionalized with an azido-tetraethylene glycol moiety which could be coupled to a complementary doxorubicin benzoyl hydrazone functionalized with a propargyl tetraethylene glycol moiety. Huisgen [3+2] cycloaddition chemistry gave the final hybrid that was evaluated for selective uptake and cytotoxicity in ER(+)-MCF-7 and ER(−)-MDA-MB-231 breast cancer cell lines. The results demonstrated that the presence of the anti-estrogenic component in the hybrid compound was critical for selectivity and cytotoxicity in ER(+)-MCF-7 human breast cancer cells as the hybrid was ~70-fold more potent than doxorubicin in inhibition of cell proliferation and promoting cell death.
Introduction
Breast cancer is the most common cancer diagnosis among women, with the majority of cases linked with the hormone responsive form of the disease.1 Because of the well established association of estradiol with estrogen receptors (ER), endocrine therapy using anti-estrogens, such as tamoxifen (TAM) and Faslodex, is the typical regimen for the treatment of hormone responsive breast cancer.2–5 Unfortunately, prolonged treatment of breast cancer patients with anti-estrogens frequently leads within 2–5 years to the emergence of recurrent disease that no longer responds to endocrine therapy. More aggressive and non-selective interventions are required that produce significant side-effects and morbidity to the patient population. Therefore, there is a continued need to develop therapeutic agents that are more effective from the very beginning and/or that do not develop resistance.
One approach to develop such agents involves combining two drugs into a single entity as a conjugate that can interact with two relevant components of the disease process. The general criteria and challenges associated with such an approach have been the subject of several reviews.6–12 In addition to fundamental concerns related to choice of targeting strategies (e.g. receptor vs. antibody), of single target versus independent/related targets, and of pathway interactions between the biological targets, one also needs to address the issues related to the chemical synthesis of such agent. In particular, the two components need to be joined in a fashion that does not compromise the activity of either, and ultimately the synthetic approach needs to be sufficiently robust to tolerate structural variants that would enhance or optimize the activity of the product.
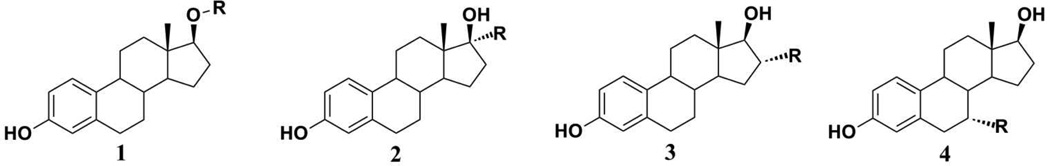
In the field of hormone responsive breast cancer, this approach typically involved linking a potent estrogen receptor targeting agent to a second component, such as an anti-metabolite, intercalating agent, anti-mitotic, alkylating agent or metal chelating group.13–28 In the case of estrogen receptor-targeted hybrids, these efforts have been almost invariably unsuccessful. To a significant extent, the lack of success can be traced to an over-reliance on chemical transformations of readily available estrogens or easily modified sites on those estrogens to prepare the target compounds. While the attachment of functional groups at the 17β-,17α-, 16α-, and 7α-positions of estradiol (Figure 1) is readily achieved through simple transformations of estradiol or its derivatives, the analysis of the crystal structures of agonist and antagonist-estrogen receptor-ligand binding domain (ER-LBD) complexes suggests that such modifications seriously impair receptor binding.29 Several examples of recent 17β-, 17α- and 16α -substituted estradiol hybrids with specific therapeutic R groups have shown low ER binding affinity.30–35 While the introduction of substituents at the 7α-position of estradiol (such as those found in the anti-estrogen Faslodex) is synthetically more challenging, the resultant products retain significant ER binding capacity and modest ER-based selectivity.36–40 X-ray crystal structures of complexes of similarly 7α-substituted ligands with ERα-LBD indicated that the steroidal scaffold was rotated around the 3–17 axis, and there was a disorder associated with helix-12, suggesting that the significant steric interactions were involved.41
Figure 1.
(1) 17-β substituted estradiol,28; (2) 17-α substituted estradiol,27,31–33; (3) 16-α substituted estradiol,30,34,35; and (4) 7-α substituted estradiol,36–40
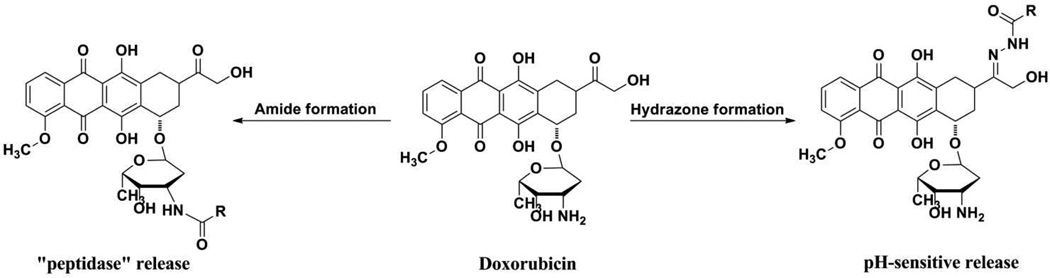
The second component of the bioconjugate involves the therapeutic agent. To address the need for more effective drugs for estrogen-responsive breast cancer, we have chosen the clinically effective anthracycline doxorubicin. While highly effective as a cytotoxic agent, its use is compromised by dose-limiting cardiotoxic side effects.42–44 Strategies to improve its clinical utility have focused on pro-drug approaches to reduce side effects and on targeted drug delivery to improve it efficacy. Some of the most promising doxorubicin derivatives involve hydrazone formation through the ketone or amide conjugation on the carbohydrate amino-group [Figure 2].42,45,46 In both approaches, intracellular processes, such as pH-dependent hydrolysis or enzymatic cleavage of the amide bond lead to free doxorubicin that generates the observed therapeutic response. While such conjugation strategies may reduce cardiotoxic effects, selective or enhanced delivery of the agent to the tumor is not improved.42
Figure 2.
Prodrug approaches in the development of doxorubicin conjugates
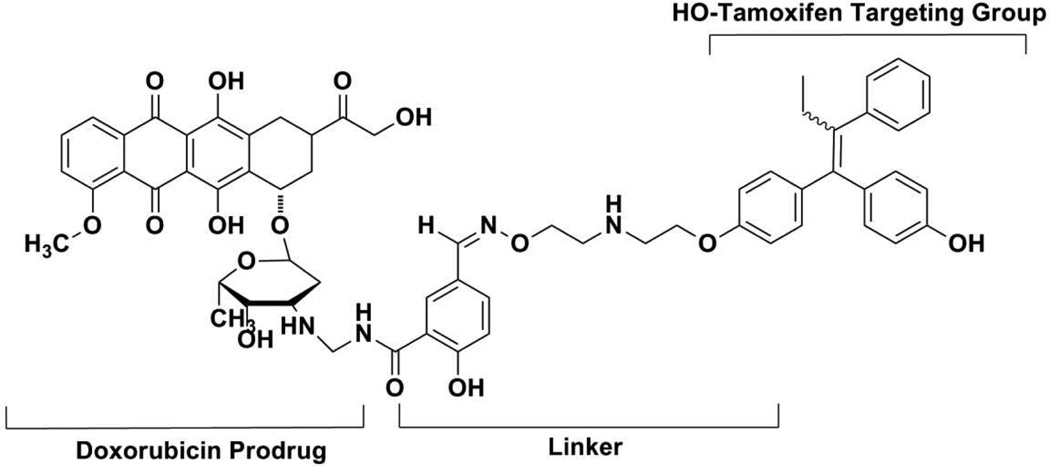
Enhanced tumor delivery of doxorubicin requires the incorporation of an appropriate targeting agent. Several examples of doxorubicin derivatives bearing tumor-selective groups, including breast cancer selective agents, have been described.47–49 For example, a non-steroidal anti-estrogen, such as, tamoxifen, (Figure 3) has been used to target doxorubicin to ER(+)-breast cancer, however, it is associated with significant problems. The parent compound, tamoxifen, has low ER affinity and exhibits substantial non-ER binding capacity. Although the hydroxylated metabolite, 4-hydroxytamoxifen, has higher ER affinity, this compound exists as a mixture of E/Z-isomers and is chemically less stable.5,50 Nevertheless, preliminary studies suggested that an enhanced and selective cytotoxicity in breast cancer cells may be achieved using a better targeting group. The conjugation of amino-sugar component doxorubicin to the amino terminus of hydroxyl tamoxifen using a releasable linker led to an increase of potency (anti-proliferative activity) in a variety of breast cancer cell lines. The investigators suggested that the targeting in this case was due to a combination of ER and anti-estrogen binding site (AEBS) effects.47,48
Figure 3.
Much of the (anti-)estrogen-doxorubicin conjugate research has focused on the targeting and therapeutic groups, however, the linking moiety is also important. For the hybrid to be effective in vitro or in vivo, the linker must be long enough to permit the ER-binding component to interact with the target protein while maintaining a stable bond with the doxorubicin. Likewise, the interaction with doxorubicin must be stable in the extracellular environment while permitting facile dissociation within the target breast cancer cells. The linker also needs to have physicochemical properties that do not compromise its formulation or biological compatibility. Therefore, our strategy in this study considered all three components hybrid drug design- the estrogen targeting component, the doxorubicin drug delivery component and the linker component that would tether the targeting, readily release mechanism and chemotherapeutic units.
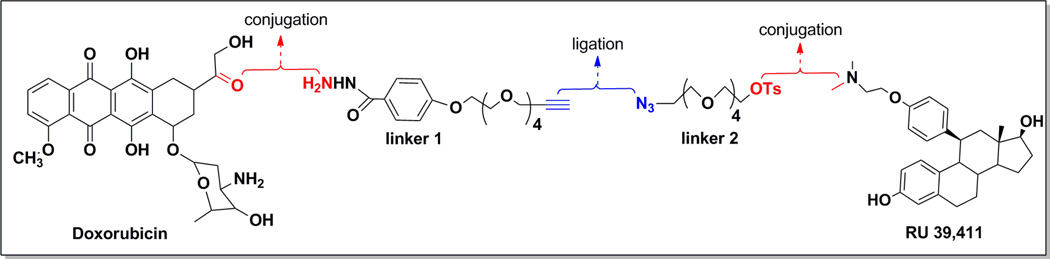
In our design of the anti-estrogen – doxorubicin conjugate, we have used a strategy involving modular assembly, i.e., each component can be developed independently and ultimately incorporated in a modular fashion. We selected an analog of steroidal anti-estrogen similar to RU39411, as our targeting component. Not only is the parent compound a pure antagonist, it possesses significantly higher ER binding affinity compared to most non-steroidal anti-estrogens such as tamoxifen and raloxifene, and it is less lipophilic.51,52 The elimination of the triarylethylene pharmacophore would also reduce interactions with the anti-estrogen binding sites not associated with the estrogen receptor. The analysis of its binding in comparison to tamoxifen-ERα-LBD complexes suggested that the ligation of linker groups via the tertiary amine should retain ER affinity, as the linker would be external to the ligand-binding pocket. The modified anti-estrogen 9 is chemically accessible via the multistep synthesis from the steroidal intermediate deltenone (as shown in the experimental section), a process with which we have significant experience.53–56 The preparation of doxorubicin hydrazones is well described as is their intracellular release under acidic conditions,45,46,57–62 however, we needed to develop a specific linker derivative to form the desired hydrazone. A key aspect of our approach involves the use of half-linkers that would be ligated in the final step to form the final conjugate. Each part would consist of a heterobifunctional tetraethylene glycol, in which one functional group consists of a “click” partner and the other functionality would interact with the targeting (anti-estrogen) or chemotherapeutic (doxorubicin) moiety. Our ultimate approach is shown in Figure 4, in which we take each component, attach it to the appropriate half-linker, and finally ligate them to form the intact bioconjugate. We hypothesize that the resultant bioconjugate should retain anti-proliferative effects comparable to doxorubicin in all cancer cell lines, but demonstrate selective anti-proliferative effects in membrane ER-expressing cancer cells. We suggest that endosomal uptake of our AE-Dox hybrid is selectively mediated through membrane ER.63 Once within the acidic cytoplasm compartment, hydrolysis of the hydrazone-Dox linkage releases the free doxorubicin generate the antiproliferative response. As our results illustrate, the final compound achieves these target properties and demonstrates selectivity towards ER(+)-breast cancer cells and promotes an enhanced cytotoxicity against those cells compared to the unmodified parent components.
Figure 4.
Strategy for Conjugate AE-Dox Synthesis
Experimental
General Methods
All solvents and reagents involved in the synthesis were reagent grade, purchased from either Sigma-Aldrich™ or Fisher Scientific, and used without further purification. Thin-layer chromatography (TLC) was done on polyester sheets pre-coated with silica gel matrix 60 F254 obtained from Sigma-Aldrich™. Separations were performed using automated flash chromatography (Argonaut FlaskMaster) or packed column chromatography with Sorbent Technologies silica gel particle size 32–63 µm and 60 Å pore size. Liquid chromatography-mass spectroscopy (LC-MS) was performed using Alliance HT -LCT Premier 2489, Waters® instrument equipped with time-of-flight (TOF) MS module. High performance liquid chromatography (HPLC) trace analysis was performed using a Waters HPLC system, equipped with a Waters 2695 binary pump, a Waters 2998 fluorescence photodiode array detector, and a XBridge™ C18 column (3.5 µm, 4.6×75 mm). 1H and 13C NMR spectra were recorded on 400 or 500 MHz Varian FT-NMR spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) by reference to proton resonances resulting from incomplete deuteration of the NMR solvent. The concentrations of test compounds were determined spectrophotometrically with a diode array UV mini 1240, Shimadzu® spectrophotometer. Ultracentrifugation of cell lysates was accomplished with Sorvall RT 6000B Refrigerated Centrifuge. All tissue culture materials were obtained from Gibco Life Technologies (Grand Island, NY) unless otherwise stated. MCF-7 and MDA-MB-231 cells were obtained from American Type Culture Collection (Rockville, MD).
Synthesis
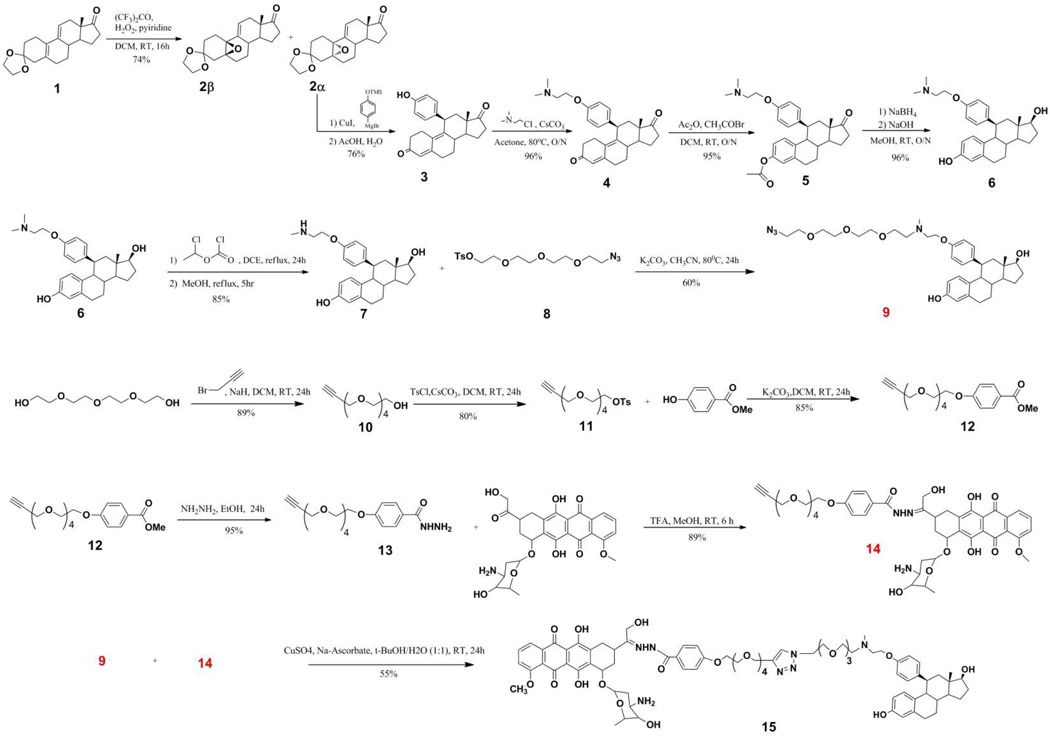
3,3-ethylenedioxy-5(10)-α-epoxy-estr-9(11)-ene 2α
To a solution of estra −5(10),9(11)-diene-3,17-dione 3-ethylenedioxy ketal 1(10.0 g, 31.8 mmol), hexafluoroacetone (0.46 mL, 3.6 mmol), and pyridine (0.23 mL, 2.86 mmol) in 10 mL of dichloromethane was added hydrogen peroxide (50%, 2.28 mL, 74 mmol) at 0 °C. After 18 h stirring at the ambient temperature, the reaction was terminated by the addition of 4 g of sodium thiosulfate in 100 mL of water and extracted with dichloromethane (3× 100 mL). The organic layer was dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure. The resulting colorless solid was triturated with diethyl ether (35 mL). The precipitate was collected by filtration and rinsed with diethyl ether (25 mL) to yield 5.23 g (15.8 mmol) 49.8 % of the 2α-isomer. The mother liquor was purified via flash chromatography to afford 2.0 g (5.8 mmol, 18.2%) of the β-isomer, and an additional 600 mg (1.8 mmol, 5.7%) of 2α-isomer. Overall 3,3-ethylenedioxy-5(10)-α-epoxy-estr-9(11)-ene-17-one (5.8 g, 17.5 mmol, 56 % yield) and 3,3-ethylenedioxy-5(10)- β-epoxy-estr-9(11)-ene-17-one (2.0 g, 5.8 mmol, 18 % yield) were obtained in a final ratio of 3:1 in favor of the α-isomer and a total yield of 74%. For 2α-isomer-1H NMR (CDCl3, 400 MHz): δ 0.88 (3H,s), 1.32-1.12 (1H,s), 2.52-2.44 (2H,m), 3.98-3.88 (4H,m), 6.06 (1H,s). 13C NMR (CDCl3, 100 MHz): δ 14.89, 22.05, 22.33, 25.23, 28.19, 31.73, 33.76, 36.05, 37.23, 40.40, 46.13, 46.81, 60.18, 61.73, 64.24, 64.45, 107.04, 125.80, 136.81, 221.37; m.p. 154°C For β-isomer-1H NMR (400 MHz, CHCl3): δ 0.87 (s, 3H), 1.32-1.12 (m, 1H), 2.52-2.44 (m, 2H), 3.98-3.88 (m, 4H), 5.87(s, 1H); C20H26O4, TOF-MS: m/z 330.18 (calcd); 353.42 [M+Na]+ (found)
11β-(4-Hydroxy-phenyl)-estra-4,9-diene-3,17-dione 3
3,3-Ethylenedioxy-5(10)-α-epoxy-estr-9-ene-17-one 2α (2.014 g, 6.1 mmol) was dissolved in anhydrous THF (15 mL) under an argon atmosphere. Copper (I) iodide (0.160 g, 0.840 mmol) was added to the solution at −10°C and stirred for 15 min. Freshly prepared Grignard reagent, (4-((trimethylsilyl)oxy)phenyl)magnesium bromide, was added dropwise in 5.0 mL aliquots. The reaction was gradually warmed to the ambient temperature and stirring was continued for 16 h. The reaction was quenched by the addition of ammonium chloride (0.8 g, 15 mmol) in 35 mL of water and 35 mL of EtOAc at 10°C. The organic layer was washed with water (2× 35 mL). The organic solvent was removed under reduced pressure and the resulting residue was dissolved in a mixture of acetic acid (14 mL) and water (6 mL). The resultant mixture was warmed at 50–60°C for 1.5 hours, after which it was diluted with ethyl acetate (20 mL). The solution was neutralized by the addition of saturated aqueous sodium bicarbonate. The organic layer was separated, washed with brine solution, dried over magnesium sulfate and evaporated to dryness to give a crude, yellow oil. Purification using silica gel column chromatography (70:30 hexane/ethyl acetate) afforded the product 3 (2.00 g, 76%) as yellow solid: 1H NMR (CDCl3, 400 MHz): δ 0.53 (3H,s), 4.38 (1H, d, J = 6.9), 5.78 (1H, s), 6.71 (2H, d), 6.97 (2H,d). 13C NMR (CDCl3, 100 MHz): δ 14.17, 21.76, 25.99, 27.06, 30.68, 35.02, 36.87, 38.14, 38.34, 39.71, 47.53, 50.77, 115.53, 122.82, 128.30, 129.83, 135.52, 145.62, 155.59, 155.99, 197.54, 217.44; C24H26O3, TOF-MS: m/z 363.19 (calcd); 384.99 [M+Na]+ (found); m.p.248°C.
11β-[4-(2-Dimethylamino-ethoxy)-phenyl]-estra-4,9-diene-3,17-dione 4
To a solution of 3 (250 mg, 0.69 mmol) and cesium carbonate (1.1 g, 3.45 mmol) in 10 mL acetone was added 2-N,N--dimethyl chloroethyl amine hydrochloride (223 mg, 2.07 mmol). The reaction mixture was heated at reflux for 16 h. The solvent was removed under reduced pressure and the residue was extracted with ethyl acetate. The organic layer was washed with water, brine solution, dried over magnesium sulfate and evaporated under reduced pressure to give a crude oil. Purification using flash chromatography yielded the product 4 (287 mg, 96%) as light yellow oil. 1H NMR (CDCl3, 400 MHz): δ 0.56 (3H, s), 1.22 – 1.41 (2H, m), 1.48 – 1.72 (2H, m), 1.91 (2H, dd, J=13.92, 6.60 Hz), 1.99 – 2.23 (6H, m), 2.28 – 2.52 (6H, m), 2.59 – 2.67 (2H, m), 2.67 – 2.89 (2H, m), 4.03 (1H, t, J=5.86 Hz), 4.38 (2H, d, J=7.33 Hz), 5.79 (1H, s), 6.84 (2H, d, J=8.79 Hz), 7.08 (2H, d, J=8.06 Hz). 13C NMR (CDCl3, 100 MHz): δ 15.6, 22.1, 26.1, 27.0, 29.6, 31.1, 32.0, 35.6, 37.0, 38.0, 39.8, 46.1, 47.9, 50.9, 54.0, 58.5, 66.1, 114.9, 123.6, 127.9, 128.0, 128.2, 130.2, 136.1, 145.2, 156.2, 157.2, 199.6; C28H35NO3, TOF-MS: m/z 433.26 (calcd); 457.99 [M+Na]+ (found)
3-Acetoxy-11β-[4-(2-dimethylamino-ethoxy)-phenyl]-estra-1,3,5(10)-triene-17-one 5
To a solution of 4 (200 mg, 0.42 mmol) in dichloromethane (10 mL) was added acetic anhydride (47 mg, 0.46 mmol) and acetyl bromide (142 mg, 1.15 mmol) at room temperature. The reaction solution was stirred for 16 h after which the product was extracted with ethyl acetate and washed with water. The organic layer was separated, dried over magnesium sulfate, and evaporated under reduced pressure to give a yellow crude oil. Column chromatography afforded the desired product 5 (207 mg, 95%). 1H NMR (CDCl3, 400 MHz): δ 0.45 (3H, s), 1.25 (2 H, s), 1.47 – 1.73 (2H, m), 1.84 – 2.02 (2H, m), 2.03 – 2.20 (6H, m), 2.22 – 2.29 (6H, m), 2.35 (3H, d, J=10.26 Hz), 2.41 – 2.64 (2H, m), 2.87 – 3.03 (2H, m), 3.07 (1H, br. s.), 4.00 – 4.22 (2H, m), 6.59 – 6.76 (3H, m), 6.86 (1H, d, J=2.20 Hz), 6.96 (2H, d, J=8.79 Hz). 13C NMR (CDCl3, 100 MHz) δ − 15.4, 21.6, 22.3, 23.1, 27.3, 30.2, 35.1, 35.5, 38.3, 40.2, 44.8, 47.8, 48.3, 48.4, 52.4, 57.4, 64.7, 64.9, 114.0, 119.4, 121.9, 128.6, 130.9, 135.6, 135.7, 137.7, 148.3, 155.7, 169.9, 176.1; C30H37NO4, TOF-MS: m/z 475.27 (calcd); 498.26 [M+Na]+ (found)
11β-[4-(2-Dimethylamino-ethoxy)-phenyl]-estra-1,3,5(10)-triene-3,17β-diol 6
To a solution of 5 (200 mg, 0.42 mmol) in methanol (5 mL) was added sodium borohydride (24 mg, 0.63 mmol) and the reaction mixture was stirred at room temperature. After 1 h, 10 N sodium hydroxide (0.025 mL, 0.25 mmol) was added and the reaction continued for 16 h. The reaction solution was poured into an ice cold mixture of ethyl acetate (20 mL) and water (20 mL), after which the organic layer was separated, washed sequentially with water and brine, and dried over magnesium sulfate. The solvent was removed under reduced pressure to give a yellow crude oil. Silica column chromatography afforded the product 6 (176 mg, 96%). 1H NMR (CDCl3, 400 MHz): δ 0.36 (3H, s), 1.17 – 1.29 (2H, m), 1.29 – 1.44 (2H, m), 1.70 (2H, d, J=8.79 Hz), 1.78 (2H, dd, J=13.18, 5.86 Hz), 1.86 – 1.99 (2H, m), 2.03 – 2.14 (2H, m), 2.27 – 2.38 (6H, m), 2.51 (3H, d, J=12.21 Hz), 2.61 – 2.67 (2H, m), 2.69 – 2.85 (2H, m), 3.68 (1H, t, J=8.06 Hz), 3.89 – 4.04 (2H, m), 6.37 (1H, dd, J=8.30, 2.44 Hz), 6.44 – 6.58 (3H, m), 6.76 (1H, d, J=8.79 Hz), 6.95 (2H, d, J=8.30 Hz). 13C NMR (CDCl3, 100 MHz): δ − 15.4, 21.6, 22.3, 23.1, 27.3, 30.2, 35.1, 35.5, 38.3, 40.2, 44.8, 47.8, 48.3, 48.4, 52.4, 57.4, 64.7, 64.9, 82.5, 114.0, 116.4, 128.6, 130.4, 131.6, 138.2, 139.1, 158.6, 155.7; C28H37NO3, TOF-MS: m/z 435.26 (calcd); 437.99 [M+Na]+ (found)
11β-[4-(2-methylamino-ethoxy)-phenyl]-estra-1,3,5(10)-triene-3,17β-diol 7
To a solution of 6 (100 mg, 0.23 mmol) in anhydrous dichloromethane (10 mL) was added α-chloroethyl chloroformate (53 µL, 0.48 mmol). The reaction mixture was stirred at 0°C for 30 minutes, and then heated at reflux for 24 h. The solvent was removed by rotary evaporation; methanol (3 mL) was added and the reaction solution was heated at reflux for 3 h. The solvent was removed by rotary evaporation to give the crude product as clear oil. Purification using silica gel chromatography gave the product 7 (82 mg, 85%) as yellow oil. 1H NMR (CDCl3, 400 MHz) δ 0.34 (3H, s), 1.20 – 1.45 (2H, m), 1.63 – 1.87 (2H, m), 1.90 – 2.18 (2H, m), 2.32 (1H, s), 2.51 (4H, m), 2.72 – 2.97 (4H, m), 3.69 (1H, t, J=8.43 Hz), 3.88 – 4.03 (2H, m), 6.39 (1H, dd, J=8.43, 2.56 Hz), 6.47 – 6.61 (3H, m), 6.79 (1H, d, J=8.06 Hz), 6.97 (2H, d, J=8.06 Hz). 13C NMR (CDCl3, 100 MHz): δ 15.4, 21.6, 22.3, 23.1, 27.3, 30.2, 35.5, 38.3, 40.2, 44.8, 47.8, 48.3, 48.4, 52.4, 57.4, 64.7, 64.9, 82.5, 114.0, 116.4, 128.6, 130.4, 131.6, 138.2, 139.1, 158.6, 155.7; C27H35NO3, TOF-MS: m/z 422.26 (calcd); 443.99 [M+Na]+ (found)
2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl tosylate 8
To a solution of tetraethylene glycol di paratoluenesulfonate (2 g, 4 mmol) in ethanol (25 mL) was added sodium azide (0.28 g, 4.3 mmol). The resulting solution was heated at 80 °C for overnight. The reaction mixture was poured into ice water (125 mL) and the product was extracted with ethyl acetate. The combined organic extracts were washed sequentially with water and brine solution, and dried over magnesium sulfate. The crude material was purified using silica gel chromatography to yield the product as clear oil 8 (1.2 g, 79%). 1H NMR (CDCl3, 400 MHz): δ 1.21 (2H, td, J=6.96, 2.93 Hz), 2.45 (3H, s), 3.40 (2H, m), 3.52–3.75 (10H, m), 3.93 (2H, m), 7.37 (2H, d), 7.79 (2H, d); C15H23N3O7S, TOF-MS: m/z 389.42 (calcd); 412.56 [M+Na]+ (found)
11β-(4-{2-[(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl)-methyl-amino]-ethoxy}-phenyl)-estra-1,3,5(10)-triene-3,17β-diol 9
To a solution of 7 (8 mg, 0.205 mmol) and potassium carbonate (43 mg, 0.31 mmol) in acetonitrile (10 mL) was added dropwise at ambient temperature under an inert atmosphere a solution of 8 (69 mg, 0.16 mmol) in acetonitrile. The solution was heated at reflux for 16 h. The reaction solvent was evaporated under reduced pressure and the resulting residue was extracted with ethyl acetate. The solvent was evaporated under reduced pressure and the crude material was purified using silica gel column chromatography to yield a light yellow oil 9 (77 mg, 60%). 1H NMR (CDCl3, 400 MHz): δ 0.31 (3 H, s), 1.18 – 1.45 (2 H, m), 1.66 – 1.81 (2 H, m), 1.99 – 2.20 (2H, m), 2.33 – 2.41 (4 H, m), 2.51 (1 H, d, J=12.46 Hz), 2.68 (2 H, t, J=5.86 Hz), 2.77 – 2.90 (2H, m), 3.34 – 3.42 (2H, m), 3.45 – 3.71 (14H, m), 3.81 (2H, t, J=4.76 Hz), 3.92 – 4.00 (4H, m), 4.00 – 4.07 (3H, m), 6.49 (1 H, dd, J=8.43, 2.56 Hz), 6.56 – 6.67 (2H, m), 6.86 (2H, d, J=8.79 Hz), 6.94 (2H, d, J=8.79 Hz). 13C NMR (CDCl3, 100 MHz): δ13.0, 17.5, 23.4, 28.2, 30.5, 30.7, 35.6, 38.4, 43.6, 43.8, 45.8, 47.6, 50.9, 52.1, 56.8, 57.4, 67.4, 69.6, 70.0, 70.3, 70.6, 70.9, 71.0, 76.9, 82.8, 112.6, 113.7, 114.8, 127.7, 130.7, 131.1, 136.0, 137.7, 155.9, 156.3; C35H50N4O6, TOF-MS: m/z 622.37 (calcd); 657.99 [M+Na]+ (found)
2-{2-[2-(2-Prop-2-ynyloxy-ethoxy)-ethoxy]-ethoxy}-ethanol 10
To a solution of tetraethylene glycol (2.00 g, 10.3 mmol) in tetrahydrofuran (10.0 mL) was slowly added sodium hydride (60% in paraffin, 580 mg, 15 mmol) at −20°C. To the reaction vessel was added dropwise at −20°C a solution of propargyl bromide (3.0 g, 22 mmol) in 5.0 mL tetrahydrofuran. The reaction mixture was stirred at −20°C for 30 minutes, allowed to warm to ambient temperature and then stirred for an additional 24 h. The reaction was partitioned between ethyl acetate (20 mL) and water (20 mL), after which the organic layer was washed with water and brine. The organic layer was dried over magnesium sulfate, filtered and the solvent was removed via rotary evaporation. The crude product was purified using silica gel column chromatography to yield 1.55 g (65%) of the product 10 as oil. 1H NMR (CDCl3, 400 MHz): δ 1.26 (1H, t, J=7.1 Hz), 2.08 – 2.09 (1H, m), 2.17 (1H, s), 2.44 (2H, t, J=2.4 Hz), 3.65 – 3.77 (14H, m), 4.21 (2H, d, J=2.4 Hz). 13C NMR (CDCl3, 100 MHz): δ 58.3, 61.5, 69.0, 70.3, 70.3, 70.5, 70.5, 70.6, 72.7, 75.1, 79.7; C11H20O5, TOF-MS: m/z 233.42 (calcd); 256.34 [M+Na]+ (found)
2-(2-(2-(2-(Prop-2-ynnyloxy)-ethoxy)-ethoxy)-ethoxy)-ethanol tosylate 11
To a solution of propargyl tetraethylene glycol 10 (1.0 g, 4.31 mmol) in CH2Cl2 (15.0 mL) was added triethylamine (1.2 mL, 8.6 mmol) and p-toluenesulfonyl chloride (0.99 g, 5.2 mmol). The reaction mixture was stirred at ambient temperature for 16 h. The solvent was under reduced pressure to give a crude product as dark oil. Separation using silica gel column chromatography gave the product 11 (1.53 g, 92%) as a yellow oil. 1H NMR (CDCl3, 400 MHz): δ. 2.45 (3H, s), 2.49 (1H, J=2.2 Hz), 3.59 – 3.64 (14H, m), 3.65 – 3.72 (2H, m), 4.13 – 4.17 (2 H, m), 4.18 (2H, J=2.2 Hz), 7.36 (2H, J=8.1 Hz), 7.79 (2H, d, J=8.1 Hz). 13C NMR (CDCl3, 100 MHz): δ 21.9, 21.9, 58.6, 68.9, 69.3, 69.5, 70.6, 70.7, 70.7, 70.8, 70.9, 74.8, 74.8, 79.9, 128.2, 130.1, 133.2, 145.0; C18H26O7S, TOF-MS: m/z 402.13 (calcd); 422.99 [M+Na]+ (found)
Methyl 4-(2-(2-(2-(2-(prop-2-ynyloxy) ethoxy)ethoxy)ethoxy)ethoxybenzoate 12
To a solution of 11 (1.0 g, 2.6 mmol) in dichloromethane (10 mL) was added dropwise a mixture of cesium carbonate (126 mg, 388 mmol) and methyl 4-hydroxybenzoate (590 mg, 3.9 mmol) in 5 mL of dichloromethane. The reaction was heated at reflux for 16 h. The reaction mixture was filtered and then concentrated under rotary evaporation. The residue was purified using silica gel column chromatography to afford the product 12 (1.1 g, 70%) as clear oil. 1H NMR (CDCl3, 400 MHz): δ 2.05 (1 H, s), 2.44 (3 H, t, J=2.56 Hz), 3.62 – 3.75 (12 H, m), 3.82 – 3.92 (2H, m), 4.09 – 4.22 (4 H, m), 6.93 (2 H, d, J=8.79 Hz), 7.98 (2 H, d, J=8.79 Hz). 13C NMR (CDCl3, 100 MHz): δ 22.6, 52.1, 52.3, 58.6, 67.8, 69.3, 69.7,70.4, 70.6, 70.8, 71.1, 74.8, 79.9, 114.4, 122.9, 131.7, 162.8, 167.0; C19H26O7, TOF-MS: m/z 366.16 (calcd); 389.10 [M+Na]+ (found)
4-(2-{2-[2-(2-Prop-2-ynyloxy-ethoxy)-ethoxy]-ethoxy}-ethoxy)-benzoic acid hydrazide 13
To a solution of 12 (200 mg, 0.55 mmol) in ethanol (10 mL) was added hydrazine hydrate (44 mg, 1.4 mmol). The solution was heated at reflux for 10 h. The solvent was removed by rotary evaporation to give a crude material that was purified by using amino column chromatography. The product 13 was isolated (144 mg, 72%) as a pale, yellow oil. 1H NMR (CDCl3, 400 MHz): δ d 1.81 (2 H, s), 2.16 (1H, s), 2.21 – 2.35 (2H, m), 3.66 – 3.81(14 H, m), 4.2 (2H, m), 6.96 (2 H, d), 7.78 (2H, d), 7.82 (1H,s). 13C NMR (CDCl3, 100 MHz): δ 67.7, 69.7, 70.1, 70.7, 71.0, 73.2, 114.5, 114.6, 125.2, 126.0, 129.0, 129.5, 144.0, 161.8, 161.9, 168.5, 169.8, 173.6 ; C18H26N2O6, TOF-MS: m/z 371.17 (calcd); 371.3 [M]+ (found)
4-(2-(2-(2-(2-(Prop-2-ynyloxy)ethoxy)ethoxy)ethoxy)ethoxybenzohydrazone--doxorubicin conjugate 14
To a solution of 12 (14 mg, 0.04 mmol) in ethanol (5 mL) was added doxorubicin hydrochloride (20 mg, .004 mmol) and trifluoroacetic acid (64.5 mg, 0.6 mmol). The reaction mixture was stirred at 20°C for 24 h, concentrated to approximately 1.0 mL and triturated with ether to yield a red precipitate. The red precipitate was collected by filtration, washed with ether, and dried under vacuum to afford the product (28.5 mg, 85%). 1H NMR (CDCl3, 400 MHz): δ 1.29 (3H, d, J=6.6 Hz), 1.81 – 1.94 (3H, m), 1.97 – 2.10 (2H,m), 2.11 – 2.22 (1H,m), 2.30 – 2.47 (1H, m), 2.89 – 3.03 (1H,m), 3.06 – 3.15 (1H,m), 3.22 – 3.37 (12H,m), 3.52 – 3.74 (3H, m), 3.78 – 3.89 (1H, m),), 4.04 (3H,s), 4.14 – 4.24 (2H,m), 4.26 – 4.33 (1H,m), 4.71 (2H, d, J=2.2 Hz), 5.03 – 5.17 (1H, m), 5.39 – 5.52 (2H, m), 6.97 – 7.13 (2H, m), 7.51 – 7.62 (1H, m), 7.74 – 7.89 (3H, m), 7.94 – 8.00 (1H, m). 13C NMR (CDCl3, 100 MHz): δ 11.6, 20.5, 28.6, 38.3, 48.1, 51.2, 61.0, 67.6, 69.4, 70.1, 70.3, 70.4, 72.4, 114.2, 117.3, 122.4, 125.8, 131.4, 140, 153.2, 155.4, 163.1, 167.4, 187.2; C45H53N3O15, TOF-MS: m/z 875.35 (calcd); 898.39 [M+Na]+ (found)
Anti-estrogen Doxorubicin Conjugate 15
To a solution of 9 (10 mg, 0.015 mmol) in 500 µL of tert-butanol/water (1:1) was added a solution of 14 (13.1 mg, 0.015 mmol) in 500 µL of tert-butanol/water (1:1). The reaction was stirred at room temperature for 30 min, followed by the addition of copper(II) sulfate pentahydrate (3.75 µL, 0.15 µmol) and (+)-sodium L-ascorbate (14.8 µL, 0.75 µmol). The reaction mixture was warmed to 40°C, stirred for 24 h, and then partitioned between water (10 mL) and dichloromethane (10 mL). The organic layer was washed with water (2×10 mL). The aqueous layers were combined; sodium chloride was added and then back extracted with dichloromethane (10 mL). The organic fractions were combined, dried over magnesium sulfate, filtered and concentrated leaving a dark red residue. The product was isolated using column chromatography (85:15 dichloromethane/methanol) to yield the product 15 (12.7 mg, 55%) as a red solid. TLC ((Si2O, 80:20 dichloromethane/methanol) Rf= 0.25: 1H NMR (CH3OH-d, 500 MHz): δ = 7.93 (d, J=7.2, 2 H), 7.89 (s, 1 H), 7.85 (s, 1 H), 7.76 (s, 1H), 7.61 – 7.67 (m, 3H), 7.56 (s, 1 H), 7.49 (s, 1 H), 7.25 (d, J=7.8 Hz, 2 H), 7.03 (d, J=8.8 Hz, 2 H), 6.99 (d, J=8.8 Hz, 2 H), 6.90 (s, 1 H), 6.83 (s, 2 H), 6.75 – 6.79 (m, 2 H), 4.36 (t, J=7.3 Hz, 1 H), 4.27 (m, 2 H), 4.16 – 4.20 (m, 1 H), 4.03 (d, J=5.9 Hz, 1 H), 3.86 – 3.90 (m, 2 H), 3.71 – 3.75 (m, 3 H), 3.63 – 3.71 (m, 28 H), 3.55 – 3.61 (m, 2 H), 3.50 – 3.54 (m, 1 H), 3.39 – 3.44 (m, 1 H), 2.39 (s, 2 H), 2.27 (s, 3 H), 1.93 (s, 1 H), 1.64 – 1.74 (m, 2 H), 1.41 – 1.50 (m, 6 H), 1.33 – 1.41 (m, 2 H), 1.30 (s, 2 H), 1.28 (br. s., 1 H), 1.26 (m, 3 H), 1.15 (m, 3 H), 1.00 ppm (m, 3 H)). C45H53N3O15, TOF-MS: m/z 1277.59 (calcd); 1299.91 [M+Na]+ (found)
High Performance Liquid Chromatography (HPLC) Trace Analysis
The compounds were analyzed with Waters HPLC system, equipped with a Waters 2695 binary pump, a Waters 2998 fluorescence photodiode array detector, and a XBridge™ C18 column (3.5 µm, 4.6×75 mm). HPLC grade acetonitrile/water/trifluroacetic acid (50/50/0.1%, v/v) was used as the mobile phase at 25°C with a flow rate of 1.0 mL min−1. Fluorescence detector was set at 254 nm for excitation and 570 nm for emission and linked to Empower III™ software for data analysis.
Cell cultures
The ER(+)MCF7 (human breast adenocarcinoma) and ER(−) MBA-MD-231 (human breast adenocarcinoma), cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM) at 37°C, 5% CO2. DMEM were supplemented with 10 % fetal bovine serum (FBS), 50 U/ml –penicillin, and 50 µg/ml streptomycin.
Cytotoxicity assays
Cells were plated at a 5×103 cells per well density in 96-well plates (Corning Inc., Corning, NY, USA). The stock solutions of free drug or drug-conjugates were prepared in DMSO and diluted in complete media before adding to cells. After 24 h, the medium was replaced with medium containing free drug or drug-conjugates. After 24 h incubation, each well was washed twice with complete media and cell survival was measured using the Cell Titer-Blue® Cell Viability Assay method. The conversion of Resaruzin to Resorufin by viable cells results in the fluorescence excitation at 550 nm. The fluorescence produced is proportional to the number of viable cell. The emitted fluorescence was measured at 590 nm (the measurement of the cytotoxicity) using a Labsystems Multiskan MCC/340 microplate reader (Labsystems and Life Sciences International, UK).
For estradiol competition assay, the cells were pretreated with 17β-estradiol (ES) (50 µM) for 1 h, followed by addition of free drug or drug-conjugates in complete media with 50 µM of ES. After 24 h incubation, cell viability was analyzed as described above.
Flow cytometry
The ER (+) MCF-7 and ER (−) MDA-MB-231 cells were grown in 12-well tissue culture plates till 70–80% confluency. The medium was removed from the wells; cells were washed with complete media and incubated with or without 50 µM ES for 1 h. After incubation, the cells were washed twice with complete media, and exposed to 0.1 µM of Dox, AE-Dox in complete media with or without 50 µM of ES. After 1 h cells were washed, trypsinized, and finally resuspended in 800 µl of 4% paraformaldeyhde in phosphate buffered saline (PBS) pH 7.4. The cell-associated fluorescence was quantified by Becton Dickinson FACScan™ (Becton Dickinson, San Jose, CA) at the emission wavelength of 580 nm (channel FL-2).The data analysis was performed using CellQuest software (Becton Dickinson). A total of 10,000 events were acquired for each sample. Data shown were derived from three separate experiments.
Fluorescence Microscopy
The ER (+) MCF-7 cells were seeded on a coverslip in six-well tissue culture plates at a concentration of 1 × 105 cells per well. After 24 h, the cells were washed twice with complete media and then incubated with 0.1 µM Dox or AE-Dox 15. After 1 h incubation, medium was removed, and cells were washed twice with sterile PBS followed by fixation of the cells with 4% paraformaldehyde (15 min at room temperature). Hoechst 33342 (1 µg/ml) was added to the cells for 15 min and cells were washed twice with sterile PBS. Cells were observed immediately on a Nikon Eclipse E400 fluorescence microscope equipped with appropriate filters for, Rhodamine and Hoechst detection. For estradiol competition assay, the cells were pretreated with 17β-estradiol (ES) (50 µM) for 1 h, followed by addition of 0.1 µM of AE-Dox in complete media with 50 µM of ES.
Results and Discussion
Synthesis
The convergent modular approach proved to be a successful strategy for assembling the target anti-estrogen doxorubicin conjugate AE-Dox 15. The starting materials were transformed to the requisite components in high yields and purity. In particular, the conversion of the readily available tetra(oligo)ethylene glycols to heterobifunctional half-linkers 8 and 13 is notable because of the possibility of subsequent variations that one can generate. If necessary, one can control the length of the spacer groups as well as the modes of ligation to address concerns not apparent at the outset of the study. In this example, the use of small tetraethylene glycol moieties facilitated the individual reactions, purifications and subsequent ligations. The conjugation the propargylated-tetraethylene glycol benzohydrazide 13 to doxorubicin to form the alkynylated intermediate 14, proceeded with good yield and remained stable under neutral and basic conditions. The preparation of the steroidal anti-estrogen 9 was readily achieved from the key starting material 1 and transformed via N-demethylation into the reactive intermediate 7. The subsequent alkylation with the azido-tetraethylene glycol tosylate 8 gave the stable component 9. The conversion into the final product 15 was achieved with good yield using the classical “click” conditions.
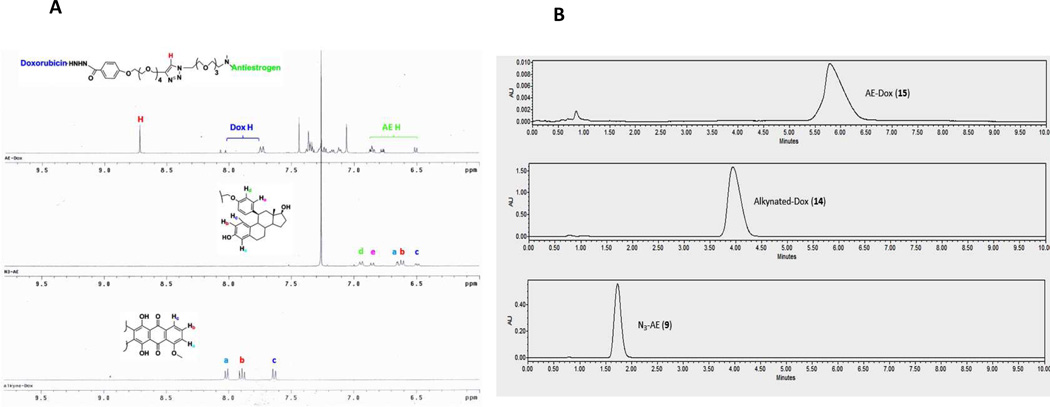
To verify the ligation of the AE-Dox conjugate 15 from its two intermediates, the azido anti-estrogen 9 and the alkynylated Dox 14, we performed the stacking 1H-NMR analysis, FT-IR and HPLC. AE-Dox formation was indicated by the signature triazole proton, shown at approximately 8.7 ppm in the 1H-NMR spectrum, and the presence of the characteristic aromatic signature protons for both Dox and AE components (Figure 5A). The FTIR spectrum supported the ligation of the two intermediates by the disappearance of the azide stretch approximately at 2100 cm−1 from compound 9, and the alkyne stretch at 2050 cm−1 (SI) from compound 14. In addition, the HPLC data (Figure 5B) showed the elution of a single peak for 15 that was distinct from the azide 15 or the alkyne 14.
Figure 5.
A) Partial stacking 1H-NMR of AE-Dox 15; B) HPLC of AE-Dox 15 and its components
Cell Studies
The preliminary evaluation of the parent compounds (Dox and RU39411), linker modified components 14 and 9, and the final AE-Dox hybrid 15 was determined using ER(+)-MCF-7 and ER(−)-MDA-MB-231 breast cancer cell lines. ER(+)-MCF-7 is a human breast adenocarcinoma cell line that overexpresses ER and is an excellent in vitro assay system to demonstrate anti-estrogenic effects,64–66 while ER(−)-MDA-MB-231 cells are insensitive to anti-hormonal interventions.67 Both cell lines are responsive to doxorubicin, such that structural modifications affecting the activity should be readily apparent. Under the conditions of the bioassay, neither cell line metabolizes doxorubicin or the anti-estrogen to a significant degree, so that any activity observed would be due primarily to the parent drug and not potential metabolites.50,68
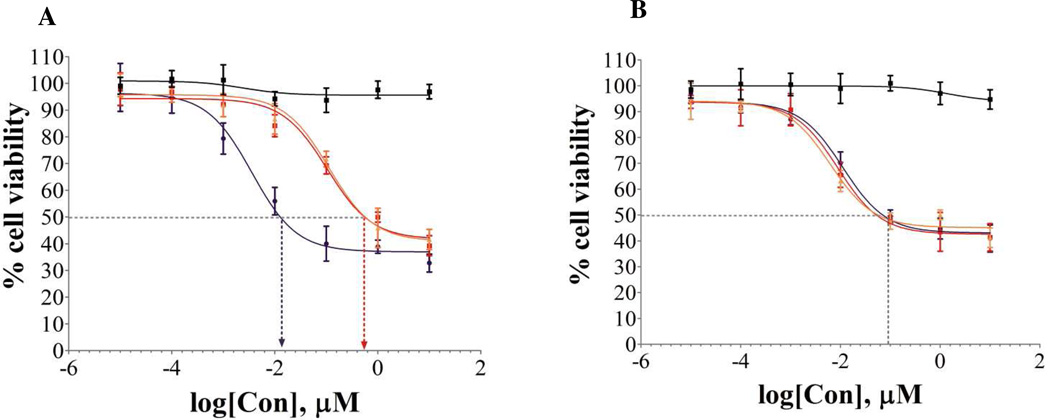
Initial cell-based assays evaluated whether the presence of the linker groups would affect the individual components as inhibitors of cell proliferation (cytotoxicity). The steroidal anti-estrogen and its azido-linker modified derivative (N3-AE 9) had no significant cytotoxic effects on ER(+)-MCF-7 cells at concentrations below 1 µM and only modest effects at 10 µM (see SI). In the same cell line, doxorubicin and its alkynyl hydrazone derivative 14 displayed statistically similar IC50 values (Table 1) and in vitro cytotoxicity profiles (results also in SI). Therefore, the introduction of the tetraethylene glycol (TEG) linker onto the amino terminus of the steroidal anti-estrogen and the TEG hydrazone linker onto the ketone of doxorubicin had no observable effect on the cytotoxic properties of the parent compounds. However, the AE-Dox conjugate 15 showed a significant enhancement of cytotoxicity (approximately 70 fold) in ER(+)-MCF-7 cells compared to the other formulations (Figure 6A). The IC50 for the AE-Dox conjugate 15 was 0.011 µM compared to 0.602 µM and 0.597 µM for the free Dox and the linker-Dox 14, respectively. In the ER(−)-MDA-MB-231 cell line (Figure 6B), the presence of the anti-estrogenic component had no significant effect. The cytotoxicity curves for all three compounds in this cell line were essentially superimposable, with IC50 values in a narrow range (0.125-0.080 µM).
Table 1.
Inhibition Concentration, IC50(nM), of various Dox compounds
| Compounds | MCF-7 | MDA-MB-231 | MCF-7 (+ES) |
|---|---|---|---|
| Dox | 602 ± 20 | 89 ± 7 | 585 ± 30 |
| Dox-Linker 14 | 597 ± 20 | 86 ± 5 | 594 ± 20 |
| AE-Dox 15 | 11 ± 6 | 90 ± 8 | 589 ± 3 |
The IC50 was estimated by using GraphPad™ 3-parameters curve fitting for 24 h drug exposure data points. All values are represented here, were evaluated in a duplicate of a triplicate count. Standard deviation was measured from the mean of six wells for each compound.
Figure 6.
Cytotoxicity of compounds 11β-AE 9 (black), Dox-linker 14-red, Dox (orange), and AE-Dox 15 (purple), in A) ER(+)MCF-7 and B) ER(−)MDA-MB-231 cell lines.
Then, we determined whether the effect of the anti-estrogenic component in the hybrid AE-Dox 15 could be reversed by the addition of estradiol (ES). Cytotoxicity of Dox and the hybrid AE-Dox 15 were analyzed in ER(+)MCF-7 cells in the presence or absence of ES. The results (Table 1) show that the enhanced cytotoxicity of the AE-Dox hybrid was completely abolished by the addition of ES whereas ES has virtually no effect on either Dox itself, or the linker-Dox 14. Therefore, it appeared that the effect was ER-dependent and not a non-specific process.
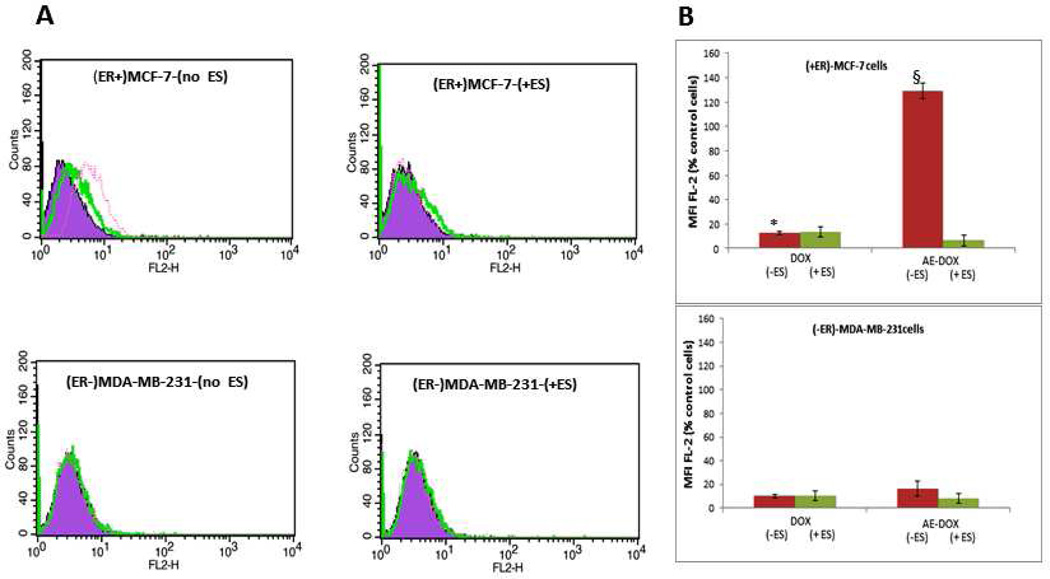
The subsequent study was undertaken both to support the ER-related effect and to identify the nature of that effect. Cell uptake/targeting of the hybrid AE-Dox 15 with ER(+)-MCF-7 and ER(−)-MDA-MB-231 cells was evaluated using fluorescent activated cell sorting (FACS), both in presence and absence of ES, as shown in Figure 7. The AE-Dox 15 demonstrated enhanced cell binding only to ER(+)-MCF-7 cells in the absence of ES. In presence of ES, cell binding of AE-Dox 15 was similar to Dox alone. Also, the ER(−)-MDA-MB-231 cells did not show any significant change in cell binding of Dox and AE-Dox 15 and, as expected, the presence or absence of ES had no effect on cell binding of the drugs. The results in Figure 7 illustrated a marked targeting effect imparted by the presence of the steroidal anti-estrogen component in the hybrid agent 15. These effects were consistent with the interaction selective for the membrane ER.
Figure 7.
The FACS analysis of ER(+)MCF-7 and ER(−)MDA-MB-231 cells. A) Histogram analysis of cells treated with 0.1 µM of Dox and AE-Dox 15 with and without ES; (purple) cells only (green) Dox treated cells and (red) AE-Dox treated cells. B) The percentage of Dox- positive cells in ER (+)MCF-7 and ER (−)MDAMB-231 cells; *P<0.01 (Dox vs AE-DOX (−ES)); §P<0.01 (AE-Dox (−ES) vs AE-Dox(+ES);
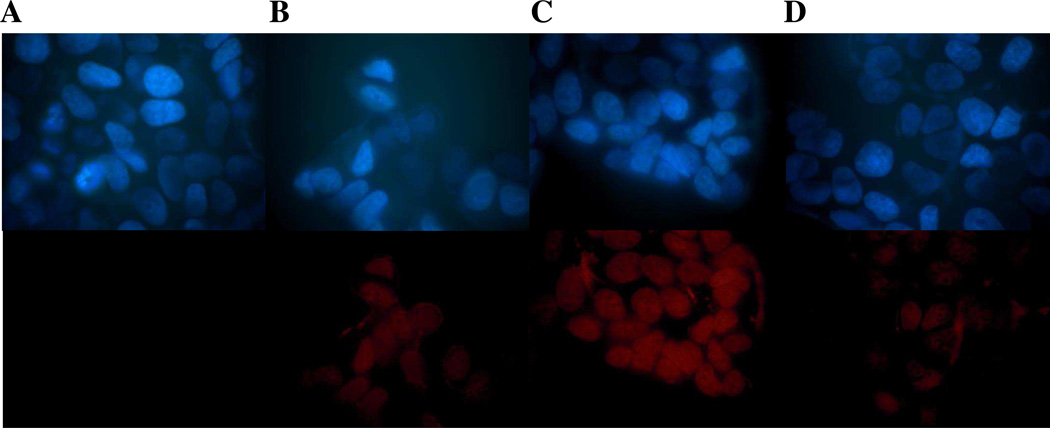
Since FACS studies do not distinguish between membrane, cytoplasmic or nuclear localization of the fluorescent group, we undertook cellular studies using the fluorescence microscopy. The results shown in Figure 8 clearly support enhanced AE-Dox 15 targeting to the ER–positive breast cancer cells. The cellular localization of the fluorescence from AE-Dox 15 and Dox was evaluated via ER(+)-MCF-7 cells in the presence (+) and absence (−) of ES. The low level of fluorescence for cells treated with Dox alone (Panel B) is associated primarily with the nucleus since the drug is known as DNA binding agent and accumulates in nucleus.69,70 AE-Dox 15 (Panel C) shows a significantly enhanced uptake within the ER(+)-MCF-7 cells, compared to Dox alone (Panel B) suggesting that the AE component of the conjugate has substantially facilitated uptake by cells. Moreover, the fluorescence appeared to be associated with nucleus, suggesting Dox should have been hydrolyzed from the targeting group coupled with the translocation of free Dox. The incubation of the cells with AE-Dox 15 and ES (Panel D) decreased the uptake of the AE-Dox (Panel B) confirming the uptake is ER-mediated. This study supported the observation for the initial cytotoxicity results in which AE-Dox 15 would generate a higher intracellular concentration of Dox and therefore more rapid cell death.
Figure 8.
Fluorescence microscopy images ER(+)MCF-7 cells treated for 1 hour with 0.1 µM of B) Dox; C) AE-Dox 15; D) AE-Dox 15 after pretreatment with 50 µM estradiol; and A) untreated cell; Hoechst fluorescent (upper panel), Red fluorescence (lower panel)
Conclusion
In this study, we have demonstrated that we can prepare the anti-estrogen-doxorubicin conjugate 15 efficiently and in high yield using our modular assembly approach. Because the components can be prepared independently, and conjugated using a simple chemistry, potential modifications of the conjugate properties are relatively easy. Initial cytotoxicity experiments demonstrated that the AE-Dox conjugate 15 was 70-fold more potent than Dox alone in ER(+)-MCF-7 cells, but equipotent compared to Dox in ER (−)-MDA-MB-231 cells. The enhanced cytotoxic effect in MCF-7 cells was reversed by pre-incubation with ES, suggesting an ER-mediated process. Subsequent FACS studies on both cell lines in the presence or absence of ES supported this hypothesis. Additional studies using fluorescence microscopy in MCF-7 cells suggested that the uptake proceded via a membrane ER-mediated effect leading to an enhanced cellular accumulation of Dox. Within the cell, the pH-sensitive hydrazone release mechanism incorporated into the conjugate leads ultimately to the elevated levels of free Dox in nucleus. Studies to further characterize the individual steps of the process are in progress.
Supplementary Material
Scheme 1.
Synthesis of Antiestrogen-Doxorubicin (AE-Dox) Conjugate 15
Acknowledgements
We acknowledge the financial support from the Northeastern University and the Department of Defense-Congressional Directed Medical Research Programs (W81XWH-10-1-0262) [K.-L.D.], Department of Energy (DE-SC0001781)[RNH], Komen Foundation (BCTR0600659)[RNH], and Public Health Service (NIH - 1R01CA121838)[VPT].
Footnotes
Supporting Information. 1H- and 13C-NMR spectra for compounds 2–15 are provided. Infrared spectra for 15, and cytotoxicity data for 9 and 14 are provided. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL. Global Cancer Facts and Figures 2007. Atlanta, Georgia: American Cancer Society; 2007. [Google Scholar]
- 2.Coezy E, Borgna J-L, Rochefort E. Tamoxifen and metabolites in MCF7 cells: Correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982;42:317–323. [PubMed] [Google Scholar]
- 3.Langan Fahey SM, Jordan VC, Fritz NF, Robinson SP, Waters D, Tormey DC. Clinical pharmacology and endocrinology of long-term tamoxifen therapy. In: Jordan VC, editor. Long-term tamoxifen treatment for breast cancer. Madison: University of Wisconsin Press; 1994. pp. 27–56. [Google Scholar]
- 4.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell (Cambridge, Mass.) 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC. Tamoxifen: A most unlikely pioneering medicine. Nat. Rev. Drug Discovery. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 6.Du D-M, Carlier PR. Development of bivalent acetylcholinesterase inhibitors as potential therapeutic drugs for Alzheimer's disease. Curr. Pharm. Des. 2004;10:3141–3156. doi: 10.2174/1381612043383412. [DOI] [PubMed] [Google Scholar]
- 7.Messer WS. Bivalent ligands for G protein-coupled receptors. Curr. Pharm. Des. 2004;10:2015–2020. doi: 10.2174/1381612043384213. [DOI] [PubMed] [Google Scholar]
- 8.Antonello A, Tarozzi A, Morroni F, Cavalli A, Rosini M, Hrelia P, Bolognesi ML, Melchiorre C. Multitarget-Directed Drug Design Strategy: A Novel Molecule Designed To Block Epidermal Growth Factor Receptor (EGFR) and To Exert Proapoptotic Effects. J. Med. Chem. 2006;49:6642–6645. doi: 10.1021/jm0608762. [DOI] [PubMed] [Google Scholar]
- 9.Morphy R, Rankovic Z. The Physicochemical Challenges of Designing Multiple Ligands. J.Med.Chem. 2006;49:4961–4970. doi: 10.1021/jm0603015. [DOI] [PubMed] [Google Scholar]
- 10.Ojima I. Guided molecular missiles for tumor-targeting chemotherapy-case studies using the second-generation taxoids as warheads. Acc. Chem. Res. 2008;41:108–119. doi: 10.1021/ar700093f. [DOI] [PubMed] [Google Scholar]
- 11.Aspland SE, Ballatore C, Castillo R, Desharnais J, Eustaquio T, Goelet P, Guo Z, Li Q, Nelson D, Sun C, Castellino AJ, Newman MJ. Kinase-mediated trapping of bi-functional conjugates of paclitaxel or vinblastine with thymidine in cancer cells. Bioorg. Med. Chem. Lett. 2006;16:5194–5198. doi: 10.1016/j.bmcl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Reddy JA, Westrick E, Santhapuram HKR, Howard SJ, Miller ML, Vetzel M, Vlahov I, Chari RVJ, Goldmacher VS, Leamon CP. Folate Receptor-Specific Antitumor Activity of EC131, a Folate-Maytansinoid Conjugate. Cancer Res. 2007;67:6376–6382. doi: 10.1158/0008-5472.CAN-06-3894. [DOI] [PubMed] [Google Scholar]
- 13.Swamy N, Purohit A, Fernandez-Gacio A, Jones GB, Ray R. Nuclear estrogen receptor targeted photodynamic therapy: selective uptake and killing of MCF-7 breast cancer cells by a C17α-alkynylestradiol-porphyrin conjugate. J. Cell. Biochem. 2006;99:966–977. doi: 10.1002/jcb.20955. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbrand G, Berger MR, Fischer J, Schneider MR, Tang W, Zeller WJ. Development of more selective anti-cancer nitrosoureas. Anti-cancer Drug Design. 1998;2:231. [PubMed] [Google Scholar]
- 15.Eisenbrand G, Fischer J, Muhlbauer K, Schied G, Schreiber J, Tang W, Zelezny O. Synthesis and characterization of steroid-linked N-(2-chloroethyl)nitrosoureas. Arch Pharm (Weinheim) 1998;322:863–872. doi: 10.1002/ardp.19893221206. [DOI] [PubMed] [Google Scholar]
- 16.Delbarre A, Oberlin R, Roques BP, Borgna JL, Rochefort H, Le PJB, Jacquemin-Sablon A. Ellipticine derivatives with an affinity to the estrogen receptor. An approach to develop intercalating drugs with a specific effect on the hormone-dependent breast cancer. J. Med. Chem. 1985;28:752–761. doi: 10.1021/jm00383a011. [DOI] [PubMed] [Google Scholar]
- 17.Devraj R, Barrett JF, Fernandez JA, Katzenellenbogen JA, Cushman M. Design, Synthesis, and Biological Evaluation of Ellipticine-Estradiol Conjugates. J. Med. Chem. 1996;39:3367–3374. doi: 10.1021/jm9602930. [DOI] [PubMed] [Google Scholar]
- 18.Kasiotis KM, Magiatis P, Pratsinis H, Skaltsounis A, Abadji V, Charalambous A, Moutsatsou P, Haroutounian SA. Synthesis and biological evaluation of novel daunorubicin-estrogen conjugates. Steroids. 2001;66:785–791. doi: 10.1016/s0039-128x(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Gacio A, Fernandez-Marcos C, Swamy N, Dunn D, Ray R. Photodynamic cell-kill analysis of breast tumor cells with a tamoxifen-pyropheophorbide conjugate. J. Cell. Biochem. 2006;99:665–670. doi: 10.1002/jcb.20932. [DOI] [PubMed] [Google Scholar]
- 20.Ali H, Ahmed N, Tessier G, Van LJE. Synthesis and biological activities of nucleoside-estradiol conjugates. Bioorg. Med. Chem. Lett. 2006;16:317–319. doi: 10.1016/j.bmcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Jones GB, Huber RS, Matthews JE, Li A. Target directed enediyne prodrugs: cytotoxic estrogen conjugates. Tetrahedron Lett. 1996;37:3643–3646. [Google Scholar]
- 22.Kuduk SD, Zheng FF, Sepp-Lorenzino L, Rosen N, Danishefsky SJ. Synthesis and evaluation of geldanamycin-estradiol hybrids. Bioorg. Med. Chem. Lett. 1999;9:1233–1238. doi: 10.1016/s0960-894x(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Strobl JS, Bane S, Schilling JK, McCracken M, Chatterjee SK, Rahim-Bata R, Kingston DGI. Design, Synthesis, and Bioactivities of Steroid-Linked Taxol Analogues as Potential Targete Drugs for Prostate and Breast Cancer. J. Nat. Prod. 2004;67:152–159. doi: 10.1021/np030296x. [DOI] [PubMed] [Google Scholar]
- 24.Bednarski PJ, Gust R, Spruss T, Knebel N, Otto A, Farbel M, Koop R, Holler E, Von AE, Schoenenberger H. Platinum compounds with estrogen receptor affinity. Cancer Treat. Rev. 1990;17:221–231. doi: 10.1016/0305-7372(90)90052-h. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee S, Das T, Chakraborty S, Samuel G, Korde A, Venkatesh M, Pillai MRA. An estradiol-conjugate for radiolabelling with 177Lu: an attempt to prepare a radiotherapeutic agent. Bioorg. Med. Chem. 2005;13:4315–4322. doi: 10.1016/j.bmc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon V, St-Germain M-E, Descoteaux C, Provencher-Mandeville J, Parent S, Mandal SK, Asselin E, Berube G. Biological evaluation of novel estrogen-platinum(II) hybrid molecules on uterine and ovarian cancers-molecular modeling studies. Bioorg. Med. Chem. Lett. 2004;14:5919–5924. doi: 10.1016/j.bmcl.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Gabano E, Cassino C, Bonetti S, Prandi C, Colangelo D, Ghiglia A, Osella D. Synthesis and characterisation of estrogenic carriers for cytotoxic Pt(II) fragments: biological activity of the resulting complexes. Org. Biomol. Chem. 2005;3:3531–3539. doi: 10.1039/b507716h. [DOI] [PubMed] [Google Scholar]
- 28.Barnes KR, Kutikov A, Lippard SJ. Synthesis, Characterization, and Cytotoxicity of a Series of Estrogen-Tethered Platinum(IV) Complexes. Chem. Biol. 2004;11:557–564. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat. Rev. Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 30.Cyrus K, Wehenkel M, Choi E-Y, Lee H, Swanson H, Kim K-B. Jostling for Position: Optimizing Linker Location in the Design of Estrogen Receptor-Targeting Protacs. ChemMedChem. 2010;5:979–985. doi: 10.1002/cmdc.201000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunanathan C, Pais A, Furman-Haran E, Seger D, Eyal E, Mukhopadhyay S, Ben-David Y, Leitus G, Cohen H, Vilan A, Degani H, Milstein D. Water-Soluble Contrast Agents Targeted at the Estrogen Receptor for Molecular Magnetic Resonance Imaging. Bioconjugate Chem. 2007;18:1361–1365. doi: 10.1021/bc700230m. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Tamrazi A, Carlson KE, Daniels JR, Lee IY, Katzenellenbogen JA. Estrogen Receptor Microarrays: Subtype-Selective Ligand Binding. J. Am. Chem. Soc. 2004;126:4754–4755. doi: 10.1021/ja039586q. [DOI] [PubMed] [Google Scholar]
- 33.Hannon MJ, Green PS, Fisher DM, Derrick PJ, Beck JL, Watt SJ, Ralph SF, Sheil MM, Barker PR, Alcock NW, Price RJ, Sanders KJ, Pither R, Davis J, Rodger A. An estrogen-platinum terpyridine conjugate: DNA and protein binding and cellular delivery. Chem.--Eur. J. 2006;12:8000–8013. doi: 10.1002/chem.200501012. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Saha P, Descoteaux C, Leblanc V, Asselin E, Berube G. Design, synthesis and biological evaluation of estradiol-chlorambucil hybrids as anticancer agents. Bioorg. Med. Chem. Lett. 2010;20:1614–1618. doi: 10.1016/j.bmcl.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Kuduk SD, Harris CR, Zheng FF, Sepp-Lorenzino L, Ouerfelli O, Rosen N, Danishefsky SJ. Synthesis and evaluation of geldanamycin-testosterone hybrids. Bioorg. Med. Chem. Lett. 2000;10:1303–1306. doi: 10.1016/s0960-894x(00)00208-0. [DOI] [PubMed] [Google Scholar]
- 36.Mitra K, Marquis JC, Hillier SM, Rye PT, Zayas B, Lee AS, Essigmann JM, Croy RG. A Rationally Designed Genotoxin that Selectively Destroys Estrogen Receptor-Positive Breast Cancer Cells. J. Am. Chem. Soc. 2002;124:1862–1863. doi: 10.1021/ja017344p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W, Sui Z. Preparation of 11-phosphorus steroid derivatives useful as progesterone receptor modulators. WO2007098381A2. World Patent. 2007 Aug 30; 2007.
- 38.Jiang X-R, Wang P, Fu X, Zhu BT. Chemical synthesis and biochemical characterization of a biotinylated derivative of 17α-estradiol with a long side chain covalently attached to its C-7α- position. Steroids. 2008;73:1252–1261. doi: 10.1016/j.steroids.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma U, Marquis JC, Dinaut AN, Hillier SM, Fedeles B, Rye PT, Essigmann JM, Croy RG. Design, synthesis, and evaluation of estradiol-linked genotoxicants as anti-cancer agents. Bioorg. Med. Chem. Lett. 2004;14:3829–3833. doi: 10.1016/j.bmcl.2004.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussey SL, He E, Peterson BR. A synthetic membrane-anchored antigen efficiently promotes uptake of antifluorescein antibodies and associated protein a by mammalian cells. J. Am. Chem. Soc. 2001;123:12712–12713. doi: 10.1021/ja017087o. [DOI] [PubMed] [Google Scholar]
- 41.Pike AC, Brzozowski AM, Walton J, Hubbard RE, Thorsell A-G, Li Y-L, Gustafsson J-A, Carlquist M. Structural insights into the mode of action of a pure antiestrogen. Structure. 2001;9:145–153. doi: 10.1016/s0969-2126(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 42.Jayaprakash S, Wang X, Heston WD, Kozikowski AP. Design and synthesis of a PSMA inhibitor-doxorubicin conjugate for targeted prostate cancer therapy. ChemMedChem. 2006;1:299–302. doi: 10.1002/cmdc.200500044. [DOI] [PubMed] [Google Scholar]
- 43.Rajski SR, Williams RM. DNA Cross-Linking Agents as Antitumor Drugs. Chem. Rev. 1998;98:2723–2796. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- 44.Skladanowski A, Konopa J. Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumour cells. Biochem. Pharmacol. 1993;46:375–382. doi: 10.1016/0006-2952(93)90512-u. [DOI] [PubMed] [Google Scholar]
- 45.Aryal S, Grailer JJ, Pilla S, Steeber DA, Gong S. Doxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriers. J. Mater. Chem. 2009;19:7879–7884. [Google Scholar]
- 46.Lee CC, Cramer AT, Szoka FC, Frachet JMJ. An Intramolecular Cyclization Reaction Is Responsible for the in Vivo Inefficacy and Apparent pH Insensitive Hydrolysis Kinetics of Hydrazone Carboxylate Derivatives of Doxorubicin. Bioconjugate Chem. 2006;17:1364–1368. doi: 10.1021/bc060117y. [DOI] [PubMed] [Google Scholar]
- 47.Burke PJ, Kalet BT, Koch TH. Antiestrogen Binding Site and Estrogen Receptor Mediate Uptake and Distribution of 4-Hydroxytamoxifen-Targeted Doxorubicin -Formaldehyde Conjugate in Breast Cancer Cells. J. Med. Chem. 2004;47:6509–6518. doi: 10.1021/jm049496b. [DOI] [PubMed] [Google Scholar]
- 48.Burke PJ, Koch TH. Design, Synthesis, and Biological Evaluation of Doxorubicin-Formaldehyde Conjugates Targeted to Breast Cancer Cells. J. Med. Chem. 2004;47:1193–1206. doi: 10.1021/jm030352r. [DOI] [PubMed] [Google Scholar]
- 49.Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA. Tamoxifen-Poly(ethylene glycol)- Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjugate Chem. 2009;20:2247–2253. doi: 10.1021/bc9002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan VC. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids. 2007;72:829–842. doi: 10.1016/j.steroids.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nique F, Van de Velde P, Bremaud J, Hardy M, Philibert D, Teutsch GJ. 11β-Amidoalkoxyphenyl estradiols, a new series of pure antiestrogens. Steroid Biochem. Mol. Biol. 1994;50:21–29. doi: 10.1016/0960-0760(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 52.Prat D, Benedetti F, Bouda LN, Girard GF. Recent developments in the synthesis of 11β-aryl-estrone derivatives. Tetrahedron Lett. 2004;45:765–768. [Google Scholar]
- 53.Hanson RN, Napolitano E, Fiaschi R. Novel high-affinity steroidal estrogenic ligands: synthesis and receptor binding of 11β-vinyl-17α-[(E/Z)-(phenylselenovinyl)]estradiols. Steroids. 1998;63:479–483. doi: 10.1016/s0039-128x(98)00052-x. [DOI] [PubMed] [Google Scholar]
- 54.Hanson RN, Napolitano E, Fiaschi R. Synthesis and Evaluation of 11β-Substituted 21-Chloro/Iodo-(17α,20E/Z)-19-norpregna-1,3,5(10),20-tetraene-3,17α-diols: High-Affinity Ligands for the Estrogen Receptor. J. Med. Chem. 1998;41:4686–4692. doi: 10.1021/jm9801051. [DOI] [PubMed] [Google Scholar]
- 55.Hanson RN. Steroidal anti-hormone hybrids with quinone antibiotics as antitumor agents. WO2010085747A1. World Patent. 2010 Jul 25;
- 56.Hanson RN, Hua EY, Labaree DC, Hochberg RB, Essigmann JM, Croy RG. AAPS Journal. 2007;9:128–147. [Google Scholar]
- 57.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Del. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Torchilin VP. Targeted Pharmaceutical Nanocarriers for Cancer Therapy and Imaging. The AAPS Journal. 2007;9:128–147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials. 2009;30:5757–5766. doi: 10.1016/j.biomaterials.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 60.Chen Q, Sowa DA, Cai J, Gabathuler R. Synthesis of Doxorubicin Conjugates Through Hydrazone Bonds to Melanotransferrin P97. Synthetic Comm. 2003;33:2377–2390. [Google Scholar]
- 61.Rodrigues PCA, Beyer U, Schumacher P, Roth T, Fiebig HH, Unger C, Messori L, Orioli P, Paper DH, Mülhaupt RM, Kratz F. Acid-Sensitive Polyethylene Glycol Conjugates of Doxorubicin: Preparation, In Vitro Efficacy and Intracellular Distribution. Bioorg. Med. Chem. 1999;7:2517–2524. doi: 10.1016/s0968-0896(99)00209-6. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues PCA, Roth T, Fiebig HH, Unger C, Mülhauptc R, Kratz F. Correlation of the acid-sensitivity of polyethylene glycol daunorubicin conjugates with their in vitro antiproliferative activity. Bioorg. Med. Chem. 2006;14:4110–4117. doi: 10.1016/j.bmc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancer. Clinical Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 64.Jordan VC. SERMs: meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 2007;99:350–356. doi: 10.1093/jnci/djk062. [DOI] [PubMed] [Google Scholar]
- 65.Kasiotis KM, Magiatis PP, Pratsinis H, Skaltsounis AL, Abadji V, Charlambous A, Moutsatsou P, Harountounian SA. Synthesis and biological evaluation of novel daunorubicin-estrogen conjugates. Steroids. 2001;66:785. doi: 10.1016/s0039-128x(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 66.Olofson RA, Martz JT, Senet JP, Piteau M, Malfroot T. A new reagent for the selective, high-yield N-dealkylation of tertiary amines: improved syntheses of naltrexone and nalbuphine. J. Org. Chem. 1984;49:2081–2082. [Google Scholar]
- 67.Mimnaugh EG, Fairchild CR, Fruehauf JP, Sinha BK. Biochemical and pharmacological characterization of MCF-7 drug-sensitive and Adr multidrug-resistant human breast tumor xenografts in athymic mice. Biochem. Pharmacol. 1991;42:391–402. doi: 10.1016/0006-2952(91)90727-m. [DOI] [PubMed] [Google Scholar]
- 68.King HD, Dubowchik GM, Mastalerz H, Willner D, Hofstead SJ, Firestone RA, Lasch SJ, Trai PA. Monoclonal Antibody Conjugates of Doxorubicin Prepared with Branched Peptide Linkers: Inhibition of Aggregation by Methoxytriethyleneglycol Chains. J. Med. Chem. 2002;45:4336–4343. doi: 10.1021/jm020149g. [DOI] [PubMed] [Google Scholar]
- 69.Taatjes DJ, Fenick DJ, Koch TH. Nuclear Targeting and Nuclear Retention of Anthracycline-Formaldehyde Conjugates Implicates DNA Covalent Bonding in the Cytotoxic Mechanism of Anthracyclines. Chem. Res. Toxicol. 1999;12:588–596. doi: 10.1021/tx990008q. [DOI] [PubMed] [Google Scholar]
- 70.Taatjes DJ, Koch TH. Growth inhibition, nuclear uptake, and retention of anthracycline-formaldehyde conjugates in prostate cancer cells relative to clinical anthracyclines. Anticancer Res. 1999;19:1201–1208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.