Abstract
It is well-established that 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) causes acute liver damage in animals and humans. The aim of this study was to identify and characterize oxidative modification and inactivation of cytosolic proteins in MDMA-exposed rats. Markedly increased levels of oxidized and nitrated cytosolic proteins were detected 12 h after the second administration of two consecutive MDMA doses (10 mg/kg each). Comparative two dimensional gel electrophoresis (2-DE) analysis showed markedly increased levels of biotin-N-methylimide (biotin-NM)-labeled oxidized cytosolic proteins in MDMA-exposed rats compared to vehicle-treated rats. Proteins in the 22 gel spots of strong intensities were identified using tandem mass spectrometry (MS/MS). The oxidatively-modified proteins identified include antioxidant defensive enzymes, a calcium-binding protein, and proteins involved in metabolism of lipids, nitrogen, and carbohydrates (glycolysis). Cytosolic superoxide dismutase was oxidized and its activity significantly inhibited following MDMA exposure. Consistent with the oxidative inactivation of peroxiredoxin, MDMA activated c-Jun N-terminal protein kinase and p38 kinase. Since these protein kinases phosphorylate anti-apoptotic Bcl-2 protein, their activation may promote apoptosis in MDMA-exposed tissues. Our results show for the first time that MDMA induces oxidative-modification of many cytosolic proteins accompanied with increased oxidative stress and apoptosis, contributing to hepatic damage.
Keywords: Cytosolic proteins, liver damage, MDMA, oxidative-modification, redox-based proteomics
1 Introduction
The amphetamine derivative (+/−)-3,4-methylenedioxymethamphetamine (MDMA1 or ecstasy) is a synthetic amphetamine analogue that is often used recreationally to achieve enhanced mood and euphoria [1,2]. The abuse of MDMA is a significant public health problem since acute exposure to MDMA is known to negatively affect physiological functions in many cells/organs and can damage various tissues such as brain, heart, liver, kidney, and testis often with fatal outcome depending on the severity of organ damage [1–4]. Furthermore, recent studies have shown that co-administration of MDMA with ethanol significantly enhances neurotoxicity and hepatotoxicity [5–8]. The hepatotoxicity and neurotoxicity induced by MDMA have been reported to be consequences of the metabolism of MDMA accompanied with its reactive intermediates such as catechols that can undergo P450-mediated metabolism to the corresponding ortho-quinones with their semiquinones [9–11]. These quinone metabolites may be conjugated with intracellular glutathione to form glutathionyl-thioester adducts, which may not be efficiently detoxified in the liver and brain. These findings suggest that the liver and brain are major target organs of MDMA-related toxicities. However, the mechanisms by which MDMA elicits adverse effects in both organs are unclear [12]. Although various factors may contribute to MDMA-induced tissue injury, our previous study demonstrated that MDMA exposure promoted oxidative modification and inactivation of many mitochondrial proteins, leading to mitochondrial dysfunction, contributing to liver damage [13]. Consequently, greater amounts of hydrogen peroxides and nitrites were produced from the mitochondria of MDMA-exposed rats compared to controls, suggesting that MDMA increases oxidative/nitrosative stress, contributing to increased oxidation of cellular macromolecules including DNA [14]. Based on the observed increase in oxidative/nitrosative stress, we hypothesized that various cytosolic proteins are oxidatively-modified and inactivated in MDMA-exposed tissues. To address this hypothesis, we characterized cytosolic proteins that were oxidized following MDMA exposure. In this study we show that many cytosolic proteins including Cu-Zn-dependent superoxide dismutase (SOD1) and peroxiredoxins (Prx), which represent thioredoxin-dependent reductases capable of removing small amounts of peroxides and/or peroxynitrite, were oxidatively-modified after MDMA exposure. Oxidative inactivation of cytosolic Prx and SOD1 reflects increased oxidative/nitrosative stress, which can directly and/or indirectly initiate cell death signaling through activation of mitogen-activated stress protein kinases (MAPK) such as c-Jun-N-terminal protein kinase (JNK) and p38 kinase (p38K) [15,16]. Our data also reveal that activation of JNK and p38K, which correlated with phosphorylation (inactivation) of Bcl-2 [17,18], may promote apoptosis (hepatotoxicity) in MDMA-exposed tissues.
2 Materials and methods
2.1 Animals and MDMA treatment
Male Sprague Dawley rats (n ≥ 6/group) were maintained in accordance with the guidelines of the National Institutes of Health. The entire protocol for this animal study was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Pharmacy. Rats were euthanized at 12 hours after the last dose of MDMA administered orally twice (10 mg/kg each on Day 1 and 2), while control rats received an equal volume of water orally on Day 1 and 2, as previously described [13]. Liver tissue from each rat was immediately excised, blotted, and stored at −80 °C until analysis.
2.2 Chemicals and other materials
MDMA, biotin-conjugated N-maleimide (biotin-NM), N-ethylmaleimide (NEM), CHAPS, anti-β-actin antibody, and DTT were purchased from Sigma Chemical (St. Louis, MO, USA) in the highest purity available. Anti-3-nitrotyrosine (3-NT) and anti-SOD1 antibody were purchased from Abcam Inc. (Cambridge, MA, USA). Specific antibodies to HRP-conjugated MAb-biotin, JNK, phospho-JNK, p38K, phospho-p38K, Bcl-XL, Bcl-2, and phospho-Bcl-2 were purchased from Cell Signaling Technology, Inc (Danvers, MA, USA). Antibodies to native Prx and hyper-oxidized Prx-SO3 were purchased from AbFrontier Co. Ltd (Seoul, Korea).
2.3 Identification of oxidized proteins by mass spectrometry
Cytosolic fractions were prepared from pooled rat livers (n ≥ 6 per group) obtained from each treatment group [13]. Labeling of oxidized proteins with biotin-NM was performed as previously described [13,19–21]. Purified biotin-NM labeled oxidized proteins bound to the streptavidin-agarose beads were washed twice with phosphate buffered saline with 1% CHAPS to remove nonspecifically bound proteins prior to their separation using two-dimensional polyacrylamide gel electrophoresis (2-DE). The resolved proteins were silver-stained, scanned, and spot intensity measured [13]. In-gel digestion of protein spots, nanoflow reversed-phase liquid chromatography–tandem mass spectrometry and bioinformatic analyses were performed as described [13,19–21].
2.4 Determination of protein carbonylation and SOD activity
The degree of protein carbonylation, an indicator of protein oxidation, was measured using the OxyELISA oxidized protein quantitation kit (Millipore, Temecula, CA, USA) following manufacturer’s instructions. Cytosolic SOD1 activity was measured using a commercial kit from Calbiochem (San Diego, CA, USA) according to the manufacturer’s protocol. Total SOD activity was determined using 0.25 mg of cytosolic proteins where one unit of enzyme activity corresponds to the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
2.5 Immunoblot analysis for measuring MAPK activity and phosphorylation of Bcl-2 protein
The activity of JNK and/or p38K was indirectly measured by immunoblot analysis using a specific antibody against phospho-JNK or phospho-p38K. The levels of Bcl-2 and phospho-Bcl-2 or Bcl-XL protein in cytosol and mitochondria were also determined by immunoblot analysis using the specific antibody against each target protein. For immunoprecipitation, cytosolic proteins (1 mg/sample) were incubated with 2 µg of anti-3-NT overnight at 4 °C with constant head-to-tail rotation using the previously described method [22,23]. Protein A/G-agarose beads were then added and the mixture incubated for 2–3 h at 4 °C. After removal of non-specifically bound proteins by washing 3 times with 1X phosphate-buffered saline plus 1% CHAPS, immunoprecipitated proteins bound to the agarose beads were incubated in SDS-sample buffer for 30 min at room temperature before being resolved on SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were incubated with a primary antibody against SOD1 overnight at 4 °C followed by HRP-conjugated secondary antibody for 1 h at room temperature. The images were developed using a kit for enhanced chemiluminescence, as described [21,24]. Densitometry analysis was performed using the software UN-SCAN-IT gel Version 6.1 [13].
2.6 Data processing and statistical analysis
All data in this report, including the carbonylation and immunoprecipitation studies, represent the results from at least three separate experiments, unless stated otherwise. Statistical analyses were performed using the Student’s t test with p<0.05 being considered statistically significant. Other methods not specifically described were performed as previously reported [24–26].
3 Results
3.1 Increased levels of oxidized and nitrated proteins in MDMA-exposed rat liver cytoplasm
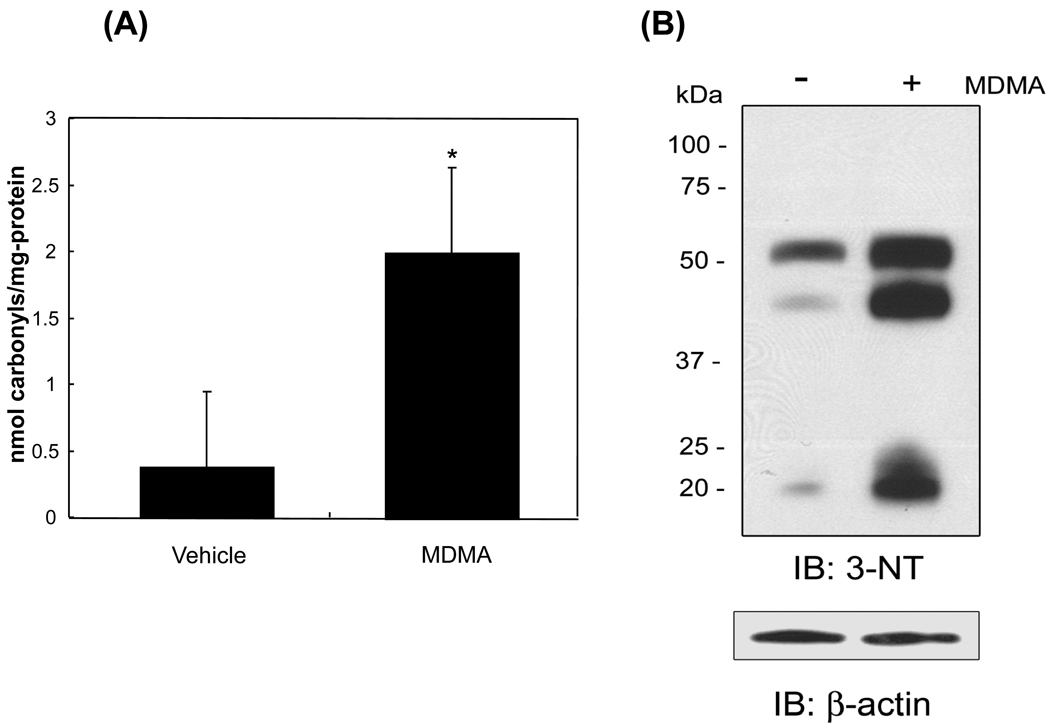
To determine whether the levels of oxidized cytosolic proteins were increased after MDMA exposure, the levels of protein carbonylation as a marker for protein oxidation were measured. The amount of carbonylated proteins increased more than 5-fold (elevated from 0.376 ± 0.57 to 2.003 ± 0.64 nmol carbonyls/mg-protein, p<0.05) after MDMA administration (Fig. 1A). To determine whether there was increased protein nitration after MDMA-exposure, the levels of 3-NT, an indicator of nitrated proteins and nitrosative stress, were also measured. The number of nitrated protein bands and intensity of 3-NT in the cytoplasm (Fig. 1B, top) were significantly greater in MDMA-exposed mice compared to vehicle-control animals, while the amounts of β-actin were similar in both samples (bottom).
Figure 1.
Increased levels of oxidized and nitrated proteins in MDMA-exposed rat liver cytoplasm. (A) The amounts of carbonylated proteins in vehicle- or MDMA-exposed rat liver cytoplasm were determined by ELISA as described in the Method Section and presented. *Significantly different from the vehicle-treated control (*p< 0.05). (B) Immunoblot analysis of nitrated cytosolic proteins. Equal amounts (20 µg/well) of cytosolic proteins from vehicle-control and MDMA-exposed rat livers were separated on a 12% SDS-PAGE gel and subjected to immunoblot analysis using a specific antibody against 3-nitrotyrosine (3-NT) (top) or β-actin (bottom).
3.2 Detection and identification of oxidatively-modified cytosolic proteins in MDMA-exposed rat livers
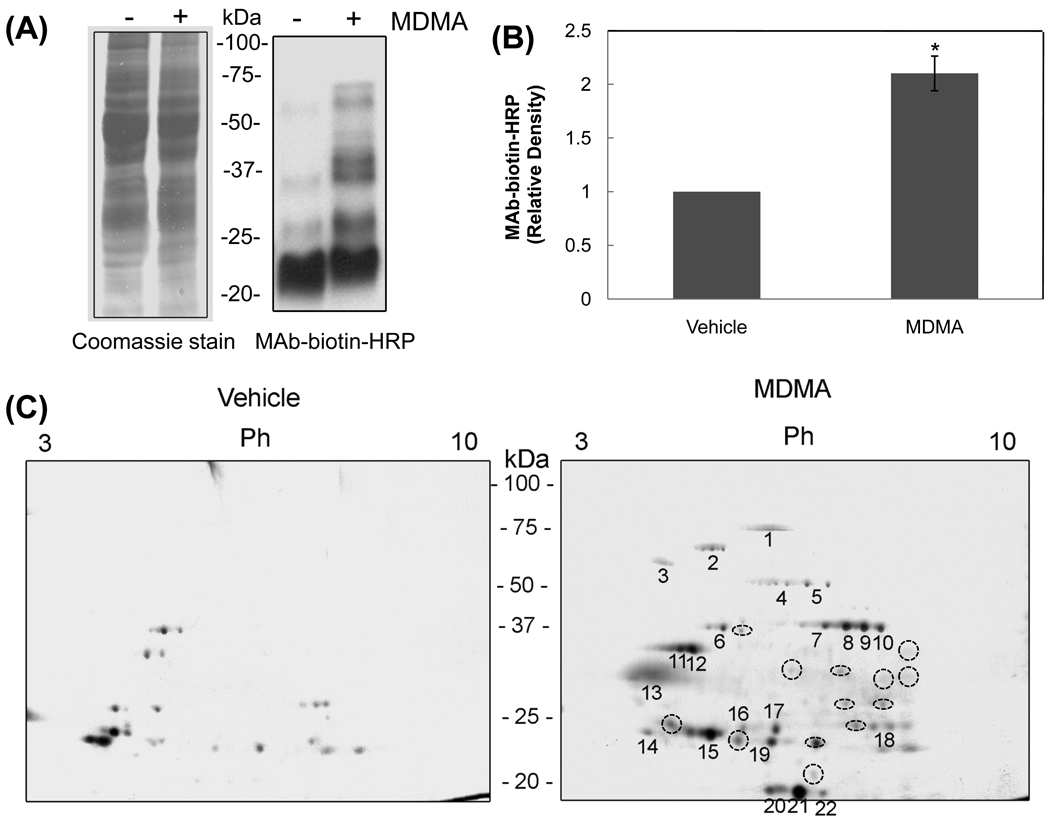
Based on increased levels of oxidized and nitrated cytosolic proteins, the oxidatively-modified proteins were identified in vehicle-control and MDMA-exposed rat livers using the redox-based Cys-targeted proteomics method [19]. Coomassie blue staining (Fig. 2A, left panel) shows that equal amounts of total proteins were analyzed for each group. The number and intensity of biotin-NM labeled proteins detected by immunoblot analysis using an HRP-conjugated-antibiotin-antibody were markedly elevated in MDMA-exposed rats (Fig. 2A, right panel, lane 2) compared to vehicle-control (lane 1). The densitometric levels of immunoreactive proteins were significantly elevated following MDMA treatment (Fig. 2B). This result indicates that MDMA exposure causes oxidation of various cytosolic rat liver proteins that were efficiently labeled with biotin-NM. Based on the increased oxidation of cytosolic proteins after MDMA exposure, we sought to identify each biotin-NM labeled protein by purification with streptavidin-agarose beads, separation by 2-DE, in-gel tryptic digestion of excised protein spots, and tandem mass spectrometry (MS/MS) [21,26]. The number and intensity of biotin-NM labeled oxidatively-modified protein spots were significantly increased in livers obtained from MDMA-treated rats (Fig. 2C, right panel) compared to those in the vehicle-control group (left panel). Table 1 shows the proteins identified from the 22 gel spots, representing oxidized cytosolic proteins with increased spot intensity in MDMA-exposed samples (Fig. 2C, right panel). In addition, the 2-D gels showed additional protein spots with increased intensities in MDMA-exposed tissues as shown in broken circles, although we were unsuccessful in identifying these proteins possibly due to their low levels of expression or a low recovery rate during in-gel trypsin digestion for MS/MS analysis. The identified oxidatively-modified proteins include enzymes/proteins involved in: antioxidant defensive (Prx6 and SOD1); lipid metabolism (fatty acid desaturase1 and arachidonate 15-lipoxygenase type II); nitrogen metabolism (arginase 1); carbohydrate metabolism (fructose-1,6-bisphosphatase 1 and α-enolase); and calcium-binding (regucalcin). We also observed acidic isoelectric point shifts of many proteins (e.g., α-enolase, aginase-1 and SOD1) without much change in their molecular size as estimated by their migration on the 2-D gels. This shift suggest these proteins are post-translationally modified (i.e., hyper-oxidation, phosphorylation, etc) as described [27]. Furthermore, smaller fragments of many proteins such as α-enolase were observed in the analysis. These results indicate spontaneous fragmentation of proteins possibly through oxidation of proline residues as previously reviewed [28]. Alternatively, these smaller protein spots may represent the degraded fragments resulting from the action of various proteolytic enzymes such as calpain [29,30] activated in MDMA-exposed tissues.
Figure 2.
Detection and identification of oxidatively-modified cytosolic proteins in MDMA-exposed rat livers. (A) Increased levels of oxidized cytosolic proteins in MDMA-exposed rat livers compared to those of vehicle-control. Cytosolic proteins from vehicle-control or MDMA-exposed rat livers were labeled with biotin-NM. Biotin-NM labeled oxidized cytosolic proteins (20 µg/well) were then separated by SDS-PAGE and stained with Coomassie blue (left) or subjected to immunoblot analysis (right) using HRP-conjugated monoclonal antibody against biotin (MAb-biotin-HRP). (B) The density of the MAb-biotin-recognized bands in each lane was determined using the gel digitizing software (UN-SCAN-IT™, Orem, UT, USA). The relative density of immunoreactive bands in MDMA-exposed sample was then calculated and compared to that in the control sample. *Significantly different from the vehicle-control (*p<0.01). (C) Comparison of oxidized cytosolic proteins by 2-DE in vehicle-control or MDMA-exposed rat livers. Oxidized cytosolic proteins (10 mg/sample) from vehicle control or MDMA-exposed rat livers were labeled with biotin-NM and then purified with streptavidin-agarose. Purified biotin-NM labeled proteins (0.25 mg/sample) were resolved by 2-DE and silver stained. Individual protein spots (spots 1–22) with differential intensities were marked with different numbers, excised out of this particular gel (pH range 3–10), in-gel digested with trypsin, and subjected to MS/MS analysis for protein identification. The protein spots in broken circles indicate that their identities could not be determined. This figure represents a typical result from two independent experiments.
Table 1.
Summary of LC-MS/MS identification of the oxidized cytosolic proteins in MDMA-exposed rat livers
| Spot No |
Oxidized cytosolic proteins in MDMA-exposed rat livers | Accession Number |
No of Peptides |
|---|---|---|---|
| 1 | Serum albumin | P02770 | 2 |
| 2 | Fatty acid desaturase 1 | Q920R3 | 1 |
| 3 | α-1-antiproteinase | P17475 | 3 |
| 4 | α-enolase | P04764 | 4 |
| 5 | α-enolase | P04764 | 5 |
| 6 | α-enolase | P04764 | 2 |
| 7 | Arginase 1 | P07824 | 10 |
| Fructose-1,6-bisphosphatase 1 | P19112 | 10 | |
| 8 | Arginase 1 | P07824 | 7 |
| 9 | Arginase 1 | P07824 | 9 |
| 10 | Arginase 1 | P07824 | 5 |
| 11 | Regucalcin (RC) | Q03336 | 3 |
| 12 | Regucalcin (RC) | Q03336 | 2 |
| 13 | Superoxide dismutase 1 (SOD1) | P07632 | 3 |
| SH2 domain binding protein 1 | Q32PZ5 | 2 | |
| 14 | α-enolase | P04764 | 2 |
| 15 | Phosphatidylethanolamine-binding protein 1 (PEBP-1) | P31044 | 2 |
| 16 | Peroxiredoxin 6 (Prx6) | O35244 | 1 |
| 17 | Peroxiredoxin 6 (Prx6) | O35244 | 3 |
| 18 | Nucleoporin GLE1 | Q4KLN4 | 2 |
| 19 | Arachidonate 15-lipoxygenase type II | Q8K4F2 | 2 |
| 20 | Superoxide dismutase 1 (SOD1) | P07632 | 4 |
| 21 | Superoxide dismutase 1 (SOD1) | P07632 | 10 |
| Catenin | Q5U302 | 3 | |
| Serine/threonine-protein kinase PCTAIRE-3 | O35832 | 2 | |
| 22 | Superoxide dismutase 1 (SOD1) | P07632 | 4 |
Biotin-NM labeled oxidized cytosolic proteins were isolated with streptavidin-agarose, washed, resolved on 2-D gels, and stained with silver. Each protein spot as indicated was cut out with a razor blade and subjected to protein identification using mass spectrometric analysis as described [13,19–21].
3.3 Inactivation of oxidatively-modified cytosolic enzymes
We hypothesized that the biological functions of many oxidized proteins might be inhibited through oxidative modifications of Cys residues within their catalytic sites as well as other critical (e.g., regulatory) sites. To test to our hypothesis, we evaluated whether the catalytic activities of Cu-Zn-dependent SOD1 and Prx were altered in MDMA-exposed rat livers since these proteins were detected in the Cys-targeted proteomics analysis (Table 1). SOD1 activity was reduced by 50% (decreased from 0.022 ± 0.002 to 0.011 ± 0.005 U/ml, p<0.05) after MDMA exposure (Fig. 3A). However, decreased SOD1 activity was not recovered even after pre-incubation of the MDMA-exposed sample with 12 mM DTT, which can reduce sulfenic acids or disulfides (including mixed disulfides with glutathione) to free sulfhydryls (Cys-SH) (data not shown). These results suggest that Cys residues of SOD1 may not be critically important to its catalysis or could be hyper-oxidized to sulfinic and/or sulfonic acids, as shown within human SOD1 [31]. Alternatively, other mechanisms such as the formation of Cys-adducts with MDMA-quinone metabolites or lipid peroxides could be a possible explanation. Furthermore, other critical amino acids such as Tyr residues within SOD1 could also be modified after MDMA exposure, as demonstrated in acetaminophen-exposed mouse tissues [23]. To directly demonstrate nitration of SOD1, nitrated proteins in the cytosol of vehicle-controls and MDMA-exposed rat livers were initially immunoprecipitated with an antibody specific to 3-NT. Immunoblot analysis using an anti-SOD1 antibody showed a single immunoreactive band in MDMA-exposed tissues but not in the vehicle-control group. These results indicate that the Tyr residue of SOD1 was nitrated along with oxidative modifications of Cys residues, possibly resulting in its inactivation (Fig. 3B).
Figure 3.
Inactivation of cytosolic Cu-Zn-SOD in MDMA-exposed rat livers. (A) Cytosolic SOD activities in vehicle-control and MDMA-exposed rat livers were determined by the method described in the Experimental Procedure and presented. *Significantly different from the vehicle-control (*p< 0.05). (B) Tyr-nitrated cytosolic proteins in the vehicle-control and MDMA-exposed rat livers were immunoprecipitated with the specific antibody against 3-NT, separated on 12% SDS-PAGE, and subjected to immunoblot analysis using an anti-SOD1 antibody.
Among the many identified oxidized cytosolic proteins, Prx was selected to test our hypothesis that oxidative modifications of critical Cys residues of target proteins lead to their inactivation, as reported previously [15,16]. As shown in Fig. 4A (left panel), the levels of total Prx in vehicle-control and MDMA-exposed rat livers were similar. However, the oxidized (inactive) form of cytosolic Prx, determined by immunoblot analysis using the anti-Prx-SO3 antibody, which specifically recognizes both sulfinic and sulfonic acids of Prx, was not observed in vehicle-control samples but was readily detected in MDMA-exposed rats, suggesting oxidation and concomitant inactivation of Prx by MDMA-mediated oxidative stress.
Figure 4.
Oxidation of Prx and subsequent activation of JNK/p38MAPK and inactivation of anti-apoptotic Bcl-2 family proteins following MDMA exposure. (A) Equal amounts (20 µg/well) of cytosolic proteins from vehicle-control or MDMA-exposed rat livers were separated on a 12% SDS-PAGE gel and subjected to immunoblot analysis using a specific antibody which recognizes either Prx (total) or oxidized Prx-SO3 (inactive form). (B,C) Equal amounts (20 µg/well) of cytosolic proteins from vehicle-control and MDMA-exposed rat livers were subjected to immunoblot analysis using the specific antibodies against JNK or phospho-JNK (B, left panel), p38K or phospho-p38K (B, right panel), Bcl-2 or phospho-Bcl-2 (C). (D) Cytosolic proteins (20 µg/lane; top) and mitochondrial proteins (20 µg/lane; bottom) were separated on a 12% SDS-PAGE gel and subjected to immunoblot analysis using a specific antibody against Bcl-XL.
3.4 Activation of MAPKs and inactivation of anti-apoptotic Bcl-2 family proteins following MDMA exposure
It is well-established that increased oxidative/nitrosative stress can activate stress-activated MAPKs such as JNK and p38K [22,32]. In addition, oxidative inactivation of Prx and SOD1, reflecting increased oxidative stress as reported in MDMA-exposed cardiac tissues [33], activates JNK and p38K but inhibits ERK activity [15,16]. Activation of JNK and p38K, coupled with inhibition of ERK, has been shown to promote apoptosis [15,22,34]. Therefore, we evaluated whether MDMA-treatment activated JNK and p38K, contributing to hepatic apoptosis. As shown in Fig. 4B, the levels of active (phosphorylated) JNK (left panels) and p38K (right panels) were increased after MDMA exposure, while the amounts of total JNK and p38K were comparable in treated and control samples.
Numerous studies show that Bcl-2 family proteins are directly involved in regulating apoptosis [35]. Activation of JNK and/or p38K is known to regulate apoptosis through phosphorylation of Bcl-2 family proteins such as Bcl-2 [17,18], Bax [22], BimEL [36], etc. as down-stream target proteins. Since we observed JNK and p38K activation, we examined whether Bcl-2 was altered (i.e., phosphorylated) in MDMA-exposed rat liver. Anti-apoptotic Bcl-2 was phosphorylated (inactivated), although its protein level was unchanged in MDMA-exposed tissues (Fig. 4C). In addition, the level of Bcl-XL, another member of the anti-apoptotic Bcl-2 family, was decreased in the cytoplasm (Fig. 4D, top) but increased in the mitochondria (bottom), suggesting its translocation (Fig. 4D) after MDMA exposure, consistent with earlier reports [37,38].
4 Discussion
MDMA abuse is widespread in young adults in the US and other European countries. Although MDMA-mediated organ toxicities are well-established, the molecular mechanisms of tissue damage are poorly understood. It has been shown that acute or chronic exposure of MDMA causes increased oxidative/nitrosative stress, which contributes to cellular damage in many tissues of humans and experimental animals [13,33,39–43]. MDMA-mediated oxidative/nitrosative stress can alter the expression of many genes and proteins resulting in significant changes in the cell signaling pathways.
Recent genetic studies revealed the gene expression changes following chronic MDMA exposure [44–46]. For example, chronic MDMA exposure altered the expression of 1028 genes including those involved in the MAPK signaling pathway, Wnt signaling pathway, long-term depression signaling pathway, etc., although the functional role of each of these major signaling pathways needs to be further established [46]. Despite these microarray-based gene expression analysis data, data concerning proteins that are up- or down-regulated by MDMA exposure is generally lacking. A recent report revealed that MDMA administration changed the levels of a few proteins in the brain (hippocampus) and these effects were still observed even 8 weeks after the MDMA exposure, suggesting a long-lasting effect of MDMA [47]. Furthermore, it is unknown which proteins are oxidatively-modified and whether these oxidized proteins contribute to MDMA-mediated oxidative/nitrosative stress and organ damage. To address these questions, we hypothesized that oxidative modifications of mitochondrial and cytosolic proteins cause mitochondrial dysfunction and inactivation of many cytosolic proteins, respectively, contributing to cell/organ damage. Based on this hypothesis, we studied functional changes in specific proteins using a Cys-targeted redox proteomics approach [19] instead of conducting global analyses of gene/protein expression by microarray [44–46] and 2-D fluorescence difference gel electrophoresis analyses [48], respectively.
In parallel to our study on oxidized mitochondrial proteins [13], the current study was focused on identifying and characterizing oxidatively-modified cytosolic proteins in MDMA-exposed rat livers. While a number of oxidatively-modified proteins were observed in vehicle-treated control animals, MDMA exposure increased the number and intensity of the oxidatively-modified cytosolic proteins (compared to those of vehicle-exposed rats). Mass-spectrometric sequence determination of each of the oxidized-protein spots on 2-D gels revealed that many cytosolic proteins/enzymes involved in the antioxidant defense system, as well as metabolic pathways including nitrogen or carbohydrate metabolism and calcium regulation, are oxidatively-modified. Our results indicated that SOD1 becomes oxidatively modified and inactivated upon MDMA exposure. A reduction in the activity (inactivation) of anti-oxidant enzymes/proteins such as SOD1 and Prx may also lead to increased levels of ROS and oxidative stress in the liver, similar to that observed in MDMA-exposed cardiac tissues [13,33]. Increased levels of ROS and nitrite/NO following MDMA exposure could produce peroxynitrite [ONOO−], a potent agent promoting S-nitrosylation of Cys residues and nitration of Tyr residues [49], as evidenced by increased oxidized (S-nitrosylated Cys residues) and nitrated (3-NT) proteins. Furthermore, increased levels of peroxynitrite could hyper-oxidize Prx (Fig. 4) and nitrate Tyr residue(s) of cytosolic SOD1 (Fig. 3). MDMA-mediated inactivation of these enzymes (current study) and activation of nitric oxide synthase [13] would not only increase oxidative/nitrosative stress, but also activate (phosphorylate) JNK and p38K, as demonstrated in cultured cells [15,16]. Stress-activated JNK and p38K are known to directly and indirectly regulate apoptosis by phosphorylating Bcl-2 family members [35]. Many members of the Bcl-2 family, some of which are downstream targets of stress-activated kinases, regulate the apoptosis process by modulating mitochondrial membrane potential and its function [22,35,36,50]. Our current result showing increased phosphorylation of Bcl-2 (Fig. 4C) in MDMA-exposed animals are not only consistent with earlier in vitro results but also indicate inactivation of Bcl-2 function [17,18,50,51]. Our Bcl-XL results (Fig. 4D) are also in agreement with those showing decreased levels of Bcl-XL in freshly-isolated hepatocytes from MDMA-exposed rats compared to those from the vehicle-control [37] as well as this protein’s translocation from the cytosol to mitochondria after exposure to various cell death stimulants such as dexamethasone and irradiation [38]. Consequently, both inactivation of Bcl-2 and translocation of Bcl-XL to the mitochondria likely contribute to increased apoptosis (hepatotoxicity) in MDMA-exposed rats.
Our data showed that multiple spots of arginanse-1, α-enolase, and SOD1 proteins are detected in the acidic regions of the isoelectric gradient with little change in their molecular sizes. These results suggest these proteins are post-translationally modified (i.e., hyper-oxidation, phosphorylation, etc), as exemplified by mitochondrial aldehyde dehydrogenase [24,26,27]. Although arginase-1 and α-enolase were shown to be phosphorylated by various protein kinases that are activated after exposure to many toxic agents including alcohol (ethanol) [52], it is unknown whether these proteins were phosphorylated by JNK or p38K, which become activated after MDMA exposure (this study). Furthermore, smaller fragments of many oxidized proteins such as α-enolase were observed in our analysis. These results indicate spontaneous fragmentation of oxidized proteins possibly through oxidation of proline residues in the protein backbones, as previously reported [20,28]. Alternatively, these low molecular weight protein spots may represent the degraded fragments produced by various proteolytic enzymes such as lysosomal proteases (e.g., calpain) [29,30] activated in MDMA-exposed tissues or through nitration of Tyr residues of these proteins followed by ubiquitin-dependent proteolytic degradation [53].
In our earlier study analyzing alcohol-exposed mouse livers, many cytosolic proteins were shown to be oxidatively-modified [20]. Oxidative modifications of chaperone proteins such as heat shock proteins and protein disulfide isomerase and other enzymes involved in the transmethylation-transsulfuration pathways were not detected under current study. Instead, we found multiple spots of oxidized arginase-1, α-enolase, and SOD1. It is presently unclear why different oxidized proteins were observed in two different conditions (i.e., alcohol versus MDMA). The levels and peak time points of ROS/RNS production and the status of various anti-oxidants could have been dissimilar in the two studies, resulting in distinctive outcomes.
In conclusion, we have demonstrated that MDMA administration results in oxidative modifications and inactivation of many cytosolic proteins involved in a variety of cellular functions: anti-oxidant defense; carbohydrate metabolism (e.g., pentose phosphate pathway and glycolysis); calcium regulation, etc. Our results also show that MDMA exposure activates the stress-related cell signaling pathway, activation of JNK and p38K, and phosphorylation of Bcl-2, possibly contributing to its inactivation and cell death in MDMA-exposed tissues. Based on the 2-DE staining, there are additional cytosolic proteins that seem to be oxidatively-modified in MDMA-exposed tissues. Oxidative modifications of cytosolic and mitochondrial proteins would inactivate some oxidized enzymes/proteins, contributing to increased oxidative/nitrosative stress and mitochondrial dysfunction, leading to MDMA-mediated liver damage especially in the presence of another abused substance such as alcohol.
Acknowledgements
This research was supported by the Intramural Research Program of National Institute of Alcohol Abuse and Alcoholism. This project has been also funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the United States Government.
Footnotes
Abbreviations used: MDMA, 3,4-methylenedioxymethamphetamine; Biotin-NM, biotin-N-maleimide; 2-DE, two-dimensional polyacrylamide gel electrophoresis; MS/MS, tandem mass spectrometry; 3-NT, 3-nitro-tyrosine; JNK; c-Jun N-terminal protein kinase; p38K, p38 mitogen-activated protein kinase; MAPK, mitogen activated protein kinase; SOD1, cytosolic superoxide dismutase 1; Prx, peroxiredoxin;
All authors have declared no conflict of interest.
References
- 1.Reid LW, Elifson KW, Sterk CE. Ecstasy and gateway drugs: initiating the use of ecstasy and other drugs. Ann Epidemiol. 2007;17:74–80. doi: 10.1016/j.annepidem.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schifano F, Corkery J, Deluca P, Oyefeso A, Ghodse AH. Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994–2003) J Psychopharmacol. 2006;20:456–463. doi: 10.1177/0269881106060147. [DOI] [PubMed] [Google Scholar]
- 3.Landry MJ. MDMA: a review of epidemiologic data. J Psychoactive Drugs. 2002;34:163–169. doi: 10.1080/02791072.2002.10399950. [DOI] [PubMed] [Google Scholar]
- 4.Nutt DJ. A tale of two Es. J Psychopharmacol. 2006;20:315–317. doi: 10.1177/0269881106064592. [DOI] [PubMed] [Google Scholar]
- 5.Izco M, Orio L, O'Shea E, Colado MI. Binge ethanol administration enhances the MDMA-induced long-term 5-HT neurotoxicity in rat brain. Psychopharmacology (Berl) 2007;189:459–470. doi: 10.1007/s00213-006-0602-1. [DOI] [PubMed] [Google Scholar]
- 6.Pontes H, Duarte JA, de Pinho PG, Soares ME, et al. Chronic exposure to ethanol exacerbates MDMA-induced hyperthermia and exposes liver to severe MDMA-induced toxicity in CD1 mice. Toxicology. 2008;252:64–71. doi: 10.1016/j.tox.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 7.Pontes H, Santos-Marques MJ, Fernandes E, Duarte JA, et al. Effect of chronic ethanol exposure on the hepatotoxicity of ecstasy in mice: an ex vivo study. Toxicol In Vitro. 2008;22:910–920. doi: 10.1016/j.tiv.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Upreti VV, Eddington ND, Moon KH, Song BJ, Lee IJ. Drug interaction between ethanol and 3,4-methylenedioxymethamphetamine ("ecstasy") Toxicol Lett. 2009;188:167–172. doi: 10.1016/j.toxlet.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiramatsu M, Kumagai Y, Unger SE, Cho AK. Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther. 1990;254:521–527. [PubMed] [Google Scholar]
- 10.Lin LY, Kumagai Y, Cho AK. Enzymatic and chemical demethylenation of (methylenedioxy)amphetamine and (methylenedioxy)methamphetamine by rat brain microsomes. Chem Res Toxicol. 1992;5:401–406. doi: 10.1021/tx00027a013. [DOI] [PubMed] [Google Scholar]
- 11.Walker TM, Davenport-Jones JE, Fox RM, Atterwill CK. The neurotoxic effects of methylenedioxymethamphetamine (MDMA) and its metabolites on rat brain spheroids in culture. Cell Biol Toxicol. 1999;15:137–142. doi: 10.1023/a:1007658501306. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa Y, Suzuki T, Tayama S, Ishii H, Ogata A. Cytotoxic effects of 3,4-methylenedioxy-N-alkylamphetamines, MDMA and its analogues, on isolated rat hepatocytes. Arch Toxicol. 2009;83:69–80. doi: 10.1007/s00204-008-0323-9. [DOI] [PubMed] [Google Scholar]
- 13.Moon KH, Upreti VV, Yu LR, Lee IJ, et al. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8:3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarenga TA, Andersen ML, Ribeiro DA, Araujo P, et al. Single exposure to cocaine or ecstasy induces DNA damage in brain and other organs of mice. Addict Biol. 2010;15:96–99. doi: 10.1111/j.1369-1600.2009.00179.x. [DOI] [PubMed] [Google Scholar]
- 15.Kang SW, Chang TS, Lee TH, Kim ES, et al. Cytosolic peroxiredoxin attenuates the activation of Jnk and p38 but potentiates that of Erk in Hela cells stimulated with tumor necrosis factor-alpha. J Biol Chem. 2004;279:2535–2543. doi: 10.1074/jbc.M307698200. [DOI] [PubMed] [Google Scholar]
- 16.Lee YM, Park SH, Shin DI, Hwang JY, et al. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 17.Brichese L, Cazettes G, Valette A. JNK is associated with Bcl-2 and PP1 in mitochondria: paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle. 2004;3:1312–1319. doi: 10.4161/cc.3.10.1166. [DOI] [PubMed] [Google Scholar]
- 18.De Chiara G, Marcocci ME, Torcia M, Lucibello M, et al. Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- 19.Suh SK, Hood BL, Kim BJ, Conrads TP, et al. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 20.Kim BJ, Hood BL, Aragon RA, Hardwick JP, et al. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250–1260. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon KH, Hood BL, Kim BJ, Hardwick JP, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 23.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon KH, Kim BJ, Song BJ. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 2005;579:6115–6120. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon KH, Abdelmegeed MA, Song BJ. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett. 2007;581:3967–3972. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon KH, Lee YM, Song BJ. Inhibition of hepatic mitochondrial aldehyde dehydrogenase by carbon tetrachloride through JNK-mediated phosphorylation. Free Radic Biol Med. 2010;48:391–398. doi: 10.1016/j.freeradbiomed.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 29.Warren MW, Larner SF, Kobeissy FH, Brezing CA, et al. Calpain and caspase proteolytic markers co-localize with rat cortical neurons after exposure to methamphetamine and MDMA. Acta Neuropathol. 2007;114:277–286. doi: 10.1007/s00401-007-0259-9. [DOI] [PubMed] [Google Scholar]
- 30.Warren MW, Zheng W, Kobeissy FH, Cheng Liu M, et al. Calpain- and caspase-mediated alphaII-spectrin and tau proteolysis in rat cerebrocortical neuronal cultures after ecstasy or methamphetamine exposure. Int J Neuropsychopharmacol. 2007;10:479–489. doi: 10.1017/S1461145706007061. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara N, Nakano M, Kato S, Yoshihara D, et al. Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J Biol Chem. 2007;282:35933–35944. doi: 10.1074/jbc.M702941200. [DOI] [PubMed] [Google Scholar]
- 32.Ghatan S, Larner S, Kinoshita Y, Hetman M, et al. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerretani D, Riezzo I, Fiaschi AI, Centini F, et al. Cardiac oxidative stress determination and myocardial morphology after a single ecstasy (MDMA) administration in a rat model. Int J Legal Med. 2008;122:461–469. doi: 10.1007/s00414-008-0262-2. [DOI] [PubMed] [Google Scholar]
- 34.Kamata H, Honda S, Maeda S, Chang L, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 36.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 37.Montiel-Duarte C, Varela-Rey M, Oses-Prieto JA, Lopez-Zabalza MJ, et al. 3,4-Methylenedioxymethamphetamine ("Ecstasy") induces apoptosis of cultured rat liver cells. Biochim Biophys Acta. 2002;1588:26–32. doi: 10.1016/s0925-4439(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 38.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darvesh AS, Yamamoto BK, Gudelsky GA. Evidence for the involvement of nitric oxide in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in the rat brain. J Pharmacol Exp Ther. 2005;312:694–701. doi: 10.1124/jpet.104.074849. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Laverty R. Role of brain nitric oxide in (+/−)3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity in rats. Brain Res. 1998;795:257–263. doi: 10.1016/s0006-8993(98)00313-8. [DOI] [PubMed] [Google Scholar]
- 42.Song BJ, Moon KH, Upreti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (Ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol. 2010;11:434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho M, Pontes H, Remião F, Bastos ML, Carvalho F. Mechanisms underlying the hepatotoxic effects of Ecstasy. Curr Pharm Biotechnol. 2010;11:476–495. doi: 10.2174/138920110791591535. [DOI] [PubMed] [Google Scholar]
- 44.Thiriet N, Ladenheim B, McCoy MT, Cadet JL. Analysis of ecstasy (MDMA)-induced transcriptional responses in the rat cortex. FASEB J. 2002;16:1887–1894. doi: 10.1096/fj.02-0502com. [DOI] [PubMed] [Google Scholar]
- 45.Xie T, Tong L, McCann UD, Yuan J, et al. Identification and characterization of metallothionein-1 and -2 gene expression in the context of (+/-)3,4-methylenedioxymethamphetamine-induced toxicity to brain dopaminergic neurons. J Neurosci. 2004;24:7043–7050. doi: 10.1523/JNEUROSCI.1626-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eun JW, Kwack SJ, Noh JH, Jung KH, et al. Transcriptomic configuration of mouse brain induced by adolescent exposure to 3,4-methylenedioxymethamphetamine. Toxicol Appl Pharmacol. 2009;237:91–101. doi: 10.1016/j.taap.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 47.van Nieuwenhuijzen PS, Kashem MA, Matsumoto I, Hunt GE, McGregor IS. A long hangover from party drugs: Residual proteomic changes in the hippocampus of rats 8 weeks after g-hydroxybutyrate (GHB), 3,4-methylenedioxymethamphetamine (MDMA) or their combination. Neuchem Int. 2010;56:871–877. doi: 10.1016/j.neuint.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Tonge R, Shaw J, Middleton B, Rowlinson R, et al. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1:377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 50.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao D, Choi S, Johnson DE, Vogel VG, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 52.Fofana B, Yao XH, Rampitsch C, Cloutier S, et al. Prenatal alcohol exposure alters phosphorylation and glycosylation of proteins in rat offspring liver. Proteomics. 2010;10:417–434. doi: 10.1002/pmic.200800969. [DOI] [PubMed] [Google Scholar]
- 53.Souza JM, Choi I, Chen Q, Weisse M, et al. Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys. 2000;380:360–366. doi: 10.1006/abbi.2000.1940. [DOI] [PubMed] [Google Scholar]