Our ability to measure nitric oxide (NO) in exhaled breath has opened a new window on the lung that improved our understanding of asthma pathobiology (1–3). Standardization of the measurement of the fraction of exhaled NO (FENO) in breath (4), followed by several large clinical and population studies, has demonstrated that, when utilized in the appropriate clinical context, FENO is useful in asthma management and provides clinicians with a new non-invasive point-of-care test to monitor airway inflammation in asthma (5). Despite all these advances, the exact role of NO in the pathogenesis of asthma remains elusive. Whether NO is beneficial through its bronchodilator and antioxidant effects or harmful by inducing inflammation remains unclear (2, 6). Understanding the complex basic biology of NO in the lung will not only improve our understanding of asthma as a disease but will also enhance our ability to use FENO to manage asthma in the clinic.
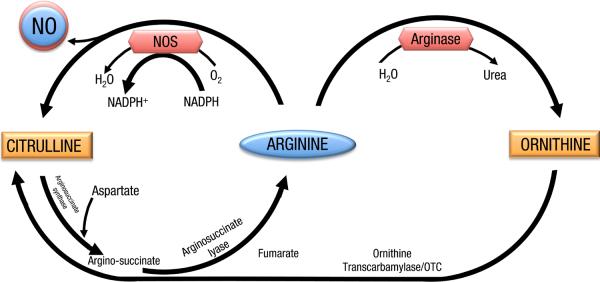
Endogenous NO is produced by nitric oxide synthases (NOS), including constitutive (neuronal, or type 1, and endothelial, or type 3) and inducible (type 2) enzymes, all isoforms of which are present in the lung (3). These enzymes utilize L-arginine and oxygen as substrates and require several cofactors including NADPH, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), calmodulin (CaM) and tetrahyrobioptrein (BH4). Arginine is a semi-essential amino acid that is also used in protein and is a substrate for other enzymes like arginases (7). Arginases compete with NOSs for arginine as a substrate and convert it to ornithine and urea (7) (Figure). The arginase pathway may be responsible (at least in part) for a peculiar concept in nitric oxide metabolism known as the “L-arginine paradox”. It refers to the phenomenon that exogenous arginine causes NO-mediated effects despite the fact that NOS may already saturated with arginine and its activity should not be affected by increasing arginine concentration. This paradox is not fully understood but several theories have been put forth to explain it based on our current understanding of arginine and NO metabolism (7) including: the compartmentalization of arginine in the cytoplasm (extra cellular arginine may be preferentially utilized by NOS within this microenvironment); the inhibitory effects of L-citrulline (cells may need extra arginine to compete with citrulline), and competition from arginase for arginine (a substrate for both enzymes) making less arginine available for NO production by NOS (8).
Figure.
A simplified schematic of the Arginine –Nitric oxide pathway(s).
To add to the complexity of NO metabolism, NO is highly reactive and once produced it can rapidly lead to the formation of several nitric oxide related end products such as nitrothyrosine, S-nitrosothiols and nitrates (2). NO is rapidly consumed by reaction with superoxide (PNAS) which is produced in asthma either spontaneously or in response to external stimuli during an exacerbation (2). Thus, nitric oxide (NO) in exhaled breath (FENO) represents a final balance between all these competing pathways leading to NO production as well as the consumption reactions that follow (2). As a free radical that reacts with oxidants and antioxidants, nitric oxide (NO) in exhaled breath (FENO) also reflects the redox state of the airway(2).
The dynamics of NO metabolism are further complicated during an asthmatic attack. Allergen challenge studies reveal multiple and sequential reactions that suggest a multifunctional role for NO in the airway (2, 9). NO rapidly consumes cytotoxic reactive oxygen species produced during the immediate asthmatic response and leads to the accumulation of less harmful reaction products. Nitrosylation reactions predominate during the late asthmatic response with accumulation of SNO, which have been proposed as safe reservoirs for removal of toxic NO derivatives. Thus, while NO may have some harmful effects in the airways as a free radical, a temporal sequence of NO participation in asthmatic airway chemical events suggests that it may also serve a protective role in the asthmatic response (2).
This complexity in NO metabolism is why any FENO value needs to be taken within the clinical and biological contexts in order to be interpreted correctly (5, 10). The same complexity, however, may be the reason why FENO can be informative and useful in so many seemingly different settings. More recently, for example, it has been shown that despite the fact that FENO levels in severe and non-severe asthma were similar, when asthma is classified based on FENO levels, a distinct asthma phenotype emerged (11). Subclassification by FENO defines severe asthma phenotypes independent of current definitions for asthma severity. In fact, asthmatics who have high FENO levels share more characteristics as compared to asthmatics with low FENO levels regardless of asthma severity as it is currently defined (12). Asthmatics with high FENO are younger and diagnosed with asthma at a younger age. They are atopic and have more eosinophilic airway inflammation, more airway reactivity, more airflow limitation, and more hyperinflation. They also seem to be less aware of asthma symptoms. Within the severe asthma group of subjects, high FENO identifies a severe asthma phenotype that has the greatest eosinophilic airway inflammation, the most severe airflow limitation, and utilizes emergent care most often (11).
It is in this context that the study by Yamamoto et al. in this issue of Clinical & Experimental Allergy adds important information to our understanding of the arginine/NO pathway and asthma severity (13). They determined the relationships of NOS expression/activation and arginase expression with asthma severity, FENO, nitrotyrosine, and eosinophilic inflammation. They confirmed that the functionality of the arginine NO pathway (measured by FENO and nitrotyrosine) was strongly related to NOSII (not arginase) levels. Interestingly, however, controlling NOSII mRNA for arginase 2 levels improved the identification of severe asthma. The investigators suggested that while NOSII expression is high in severe asthma, and may explain the high levels of FENO in these patients, factors controlling arginase expression significantly improve differentiation of severity.
This study adds to the growing evidence of the importance and the complexity of the arginine-NO pathway in asthma. This accumulating knowledge will hopefully allow us to fine-tune or even revise altogether the way we grade asthma severity and define asthma phenotypes. Our classifications can be based more on the biology of the disease and biomarkers (like FENO) that reflect this biology and not only on the secondary clinical and physiologic manifestation.
Acknowledgements
Dr Dweik is supported by the following grants: HL081064, HL107147, HL095181, and RR026231 from the National Institutes of Health (NIH), and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD).
Footnotes
This editorial discusses the findings of the paper in this issue by Yamamoto et al (ref 13, ppXXX)
References
- 1.Dweik RA. The promise and reality of nitric oxide in the diagnosis and treatment of lung disease. Cleve Clin J Med. 2001;68(6):486, 8, 90, 93. doi: 10.3949/ccjm.68.6.486. [DOI] [PubMed] [Google Scholar]
- 2.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98(5):2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, et al. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101(3):660–6. doi: 10.1172/JCI1378. PMCID: 508610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 5.Dweik RABP, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozkan M, Dweik RA. Nitric Oxide and Airway Reactivity. Clinical Pulmonary Medicine. 2001;8(4):199–206. [Google Scholar]
- 7.Sy BMC, Dweik EE, Dweik RA. Arginine and Nitric Oxide. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10 ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. [Google Scholar]
- 8.Dweik RA. The lung in the balance: arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L15–7. doi: 10.1152/ajplung.00322.2006. [DOI] [PubMed] [Google Scholar]
- 9.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol. 2003;95(1):436–40. doi: 10.1152/japplphysiol.01127.2002. discussion 5. [DOI] [PubMed] [Google Scholar]
- 10.Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest. 2008;133(4):837–9. doi: 10.1378/chest.07-2743. [DOI] [PubMed] [Google Scholar]
- 11.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162(6):2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Tochino Y, Chibana K, Trudeau JB, Holguin F, Wenzel SE. Nitric oxide and related enzymes in asthma: Relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2012;42(this issue) doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]