Abstract
Background and Purpose
We investigated whether brain arteriovenous malformation (bAVM) silent intralesional microhemorrhage (SIM), i.e., asymptomatic bleeding in the nidal compartment, might serve as a marker for increased risk of symptomatic intracranial hemorrhage (ICH). We evaluated two markers to assess the occurrence of SIM: neuroradiological assessment of evidence of old hemorrhage (EOOH)— imaging evidence of bleeding before the outcome events, and hemosiderin positivity in H&E-stained paraffin block sections.
Methods
We included cases from a bAVM database with recorded neuroradiological data or available surgical paraffin blocks. Using two endpoints, index ICH and new ICH after diagnosis (censored at treatment, loss to follow-up, or death), we performed logistic or Cox regression to assess EOOH and hemosiderin positivity, adjusting for age, sex, deep-only venous drainage, maximal bAVM size, deep location, and associated arterial aneurysms.
Results
EOOH was present in 6.5% (n=975) of patients and highly predictive of index ICH (p<0.001; OR=3.97; 95% CI, 2.1-7.5), adjusting for other risk factors. In a multivariable model (n=643), EOOH was an independent predictor of new ICH (HR=3.53; 95% CI=1.35-9.23; p=.010). Hemosiderin positivity was found in 36.2% (29.6% in unruptured; 47.8% in ruptured; p=.04), and associated with index ICH in univariate (OR=2.18; 95%CI 1.03-4.61; p=.042; n=127) and multivariable models (OR=3.64; 95% CI=1.11-12.00; p=.034; n=79).
Conclusions
The prevalence of SIM is high and there is evidence for an association with both index and subsequent ICH. Further development of means to detect SIM during bAVM evaluation may present an opportunity to improve risk-stratification, especially for unruptured bAVMs.
Keywords: brain arteriovenous malformation, hemorrhage, risk factor, survival, brain microbleed, hemosiderin
Optimal management of brain arteriovenous malformation (bAVM) is critically dependent on accurate assessment of the competing risks of interventional treatment versus those of the natural history of the disease. The strongest and most widely accepted risk factor is clinical presentation with a symptomatic ICH; other risk factors such as age at diagnosis, deep-only venous drainage and deep location, are weaker in comparison.1, 2 Among patients who present with an unruptured lesion—roughly half of all detected cases—additional risk factors are needed to further discriminate risk of future symptomatic ICH.
Although the occurrence of clinically “silent” hemorrhages in bAVM patients has been recognized for some time,3-5 use of this information for risk-stratification has not been developed. Because clinically symptomatic ICH at presentation (index ICH) is such a strong risk factor for future ICH, these silent intralesional microhemorrhages (SIM) may represent the same biological phenomenon and also signal increased risk for clinical ICH. Importantly, they may be amenable to detection by MR sequences sensitive to iron, as proposed for other disease states.6, 7
Our interest in SIM began when we noted that our animal model of the bAVM phenotype was associated with microbleeding.8, 9 In initial comparisons to human samples, we found a much higher incidence of hemosiderin and Prussian Blue positivity than expected, even in unruptured cases. To further explore the phenomenon, we examined a variable in our bAVM study project database1, as described in the Joint Writing Group proposed reporting terminology10: neuroradiological evidence of old hemorrhage (EOOH). The surprising strength of the EOOH effect that we noted prompted us to systemically review histopathological material.
Specifically, we investigated whether two markers of SIM that occurred prior to diagnosis were associated with a higher risk of symptomatic intracranial hemorrhage (ICH), i.e., studying the association of ICH with (a) EOOH, and (b) the presence of hemosiderin in archived paraffin blocks. We addressed the question of whether SIM, i.e., clinically-silent episodes of bleeding in the nidal compartment, might signal increased risk of clinically symptomatic ICH.
Methods
All patients included are part of the UCSF Brain AVM Study Project registry. Those enrolled prospectively since 2000 gave informed consent; cases between 1992 and 2000 were reviewed retrospectively. This work was approved by the UCSF Committee on Human Research.
Neuroradiological Review
A standardized neuroradiological data collection form following the Joint Writing Group guidelines10 was completed by an attending neuro-interventional radiologist. Available imaging data generally included tomographic imaging from an outside facility, where the presumptive diagnosis was made, and four-vessel angiograms performed at UCSF.
We included only cases for which information on evidence of old hemorrhage (EOOH) was recorded, defined as CT or MR (generally T1- or T2-weighted sequences) evidence of bleeding prior to diagnosis. MR evidence included signal loss consistent with hemosiderin as well as indirect evidence of old hemorrhage, i.e., encephalomalacia adjacent to the lesion consistent with a prior hematoma or calcification; CT was only useful for assessing parenchymal calcification and encephalomalacia.
Tissue Samples
We selected a series of paraffin blocks collected from 1992 and 2011, enriched for (a) unruptured cases, and (b) those at the extremes of age (≤15 years and ≥60 years), to examine age-dependency seen in other forms of microbleeds, such as lobar ICH.11 Five μm sections were stained with hematoxylin and eosin (H&E).
Blinded to patient history, two neuropathology reviewers (TS and TT) used a four-point scale (none; small; moderate; large) to gauge the relative amount of hemosiderin, using birefringent as criteria, or brownish material seen within the vascular wall or stromal tissue between vascular elements. Staining visualized only on the luminal surface, within vessel lumens, or inside of a macrohemorrhage and adjacent fibrinous material, was not counted. Macrophage infiltration was assessed on a five-point scale (none, minimal, focal, marked, extensive). For statistical analysis, both scales were dichotomized into absence or presence.
An exploratory experiment was performed to see how well ex vivo MR imaging correlated to histological evidence of prior hemorrhage (see Supplemental Figures S1 and S2, http://stroke.ahajournals.org).
Statistical Analyses
For neuroradiological data, patients with and without EOOH were compared using descriptive statistics, including t tests for continuous variables and chi-squared tests for categorical variables. Although a range of risk factors has been described as being associated with both index and follow-up ICH, we pre-defined a limited set for this analysis, including age at diagnosis (decade), sex, deep-only venous drainage, maximal bAVM size (cm), deep location, and associated arterial aneurysms (either intranidal or extranidal flow-related arterial aneurysms).2
Analysis was carried out in two stages. The first stage examined the relationship between initial clinical presentation with symptomatic ICH (index ICH) and EOOH. Both univariate and multivariable logistic regression analysis was performed using index ICH as the outcome and EOOH as the primary predictor. We chose to examine a multivariable model with inclusion of all pre-defined ICH risk factor variables, irrespective of univariate significance.
The second stage examined the relationship between EOOH and its effect on the time to subsequent ICH after diagnosis in the natural course prior to any treatment. We performed Cox proportional hazards analysis of time to first ICH, censoring patients at treatment, death, or last follow-up. Kaplan-Meier survival curves and log-rank tests assessed hemorrhage-free survival for patients with and without EOOH. Both univariate and multivariable Cox proportional hazards models were used, similarly including all putative risk factors for ICH. Sensitivity analyses included exclusion of cases with a prior history of stroke.
Because we believe that EOOH and index ICH are in the same causal pathway, we did not include index ICH in the multivariable model. However, as a sensitivity analysis, we performed a mediational analysis. We constructed a separate multivariable model substituting index ICH for EOOH and then an additional model with both index ICH and EOOH, and their interaction.
For the hemosiderin analysis of paraffin block sections, univariate and multivariable logistic regression analyses were performed similar to the above, but using hemosiderin positivity as the primary predictor. Sensitivity analyses included removing cases that underwent pre-operative embolization and/or had a history of prior stroke and an exploration of the effect of delay between surgical harvest and either diagnosis or last ICH event.
Statistical analyses used Intercooled Stata version 12. A p value of p<.05 was taken as the level of significance.
Results
Association of EOOH with Index ICH
Baseline characteristics (n= 975) are shown in Table 1; EOOH was present in 6.5% of patients. Representative images of cases scored positive for EOOH are shown in Figure 1. The fraction of presentation with index ICH was greater in the EOOH group (76 vs 42%; p<0.001). Other characteristics did not differ (p>0.05) between EOOH groups, the exception being a history of remote stroke (7/15 histories consistent with hemorrhagic stroke). Multivariable logistic regression results are shown in Table 2 and univariate results in Supplemental Table S1 (see http://stroke.ahajournals.org). The multivariable model showed that EOOH was highly predictive of index ICH (OR=4.42, 95% CI: 2.10-7.50, p<0.001). Age, deep-only venous drainage, bAVM size, deep location and associated arterial aneurysm remained highly predictive of index ICH in a multivariable model.
Table 1.
Baseline characteristics of those with and without evidence of prior hemorrhage (EOOH). Frequencies shown as number (%) unless indicated.
| Characteristics | EOOH Yes (n = 63) |
EOOH No (n = 912) |
Total (n = 975) |
P Value |
|---|---|---|---|---|
| Demographic | ||||
| Gender | 0.536 | |||
| Male | 33 (51) | 441 (48) | 474 (49) | |
| Female | 30 (49) | 471 (52) | 501 (51) | |
| Age at diagnosis, decades | 3.7 ± 1.8 | 3.8 ± 1.8 | 3.8 ± 1.8 | 0.842 |
| Ethnicity | 0.414 | |||
| Caucasian | 20 (36) | 408 (48) | 428 (47) | |
| Black | 4 (7) | 55 (6) | 59 (6) | |
| Hispanic | 19 (34) | 186 (22) | 205 (23) | |
| Asian | 6 (11) | 91 (11) | 97 (11) | |
| American Indian | 0 (0) | 9 (1) | 9 (1) | |
| Unknown | 8 (13) | 104 (12) | 112 (12) | |
| Mixed Race | 0 (0) | 2 (< 1) | 2 (< 1) | |
| Clinical | ||||
| Initial Presentation Hemorrhage | < 0.001 | |||
| Yes | 48 (76) | 383 (42) | 431 (44) | |
| No | 15 (24) | 529 (58) | 544 (56) | |
| Venous Drainage | 0.149 | |||
| Deep-only | 16 (27) | 166 (19) | 182 (19) | |
| Not Exclusively Deep | 44 (73) | 706 (81) | 750 (81) | |
| Eloquence | 0.927 | |||
| Yes | 33 (57) | 494 (58) | 527 (58) | |
| No | 25 (43) | 365 (42) | 390 (42) | |
| Deep Location | 0.767 | |||
| Yes | 11 (18) | 148 (17) | 159 (16) | |
| No | 51 (82) | 760 (83) | 811 (84) | |
| Aneurysm Associated Aneurysm | 0.161 | |||
| Yes | 14 (25) | 285 (34) | 299 (34) | |
| No | 42 (75) | 550 (66) | 592 (66) | |
| Embolization | 0.796 | |||
| Yes | 25 (40) | 347 (38) | 372 (38) | |
| No | 38 (60) | 565 (62) | 603 (62) | |
| Prior History of Hemorrhage | < 0.001 | |||
| Yes | 8 (13) | 7 (1) | 15 (2) | |
| No | 55 (88) | 905 (99) | 960 (98) | |
| Maximal AVM Size (cm) | 2.8 ± 1.5 | 2.9 ± 1.6 | 2.9 ± 1.6 | 0.520 |
Figure 1.
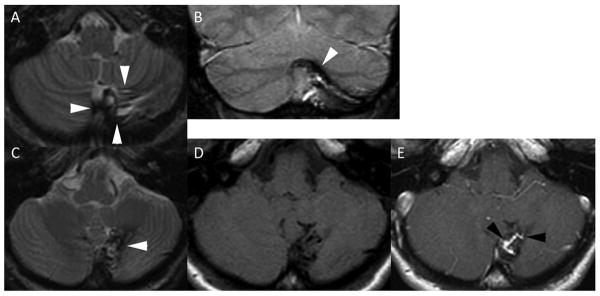
Representative case scored positive for “evidence of old hemorrhage (EOOH)”. Cerebellar AVM with evidence of prior hemorrhage. A 42 year old woman was diagnosed with an asymptomatic AVM of the cerebellum. MRI demonstrates encephalomalacia of the inferior vermis and medial and inferior left cerebellar hemisphere. Axial T2 weighted fast spin echo (A, C) and coronal gradient echo (B) images reveal low signal consistent with hemosiderin (white arrowheads) staining the brain surrounding the AVM. The AVM itself is evident as a linear focus of enhancement (compare panel D axial T1 spin echo to panel E axial T1 spin echo post gadolinium) between black arrowheads in the center of the hemosiderin stained brain parenchyma.
Table 2.
Multivariable associations for association of EOOH with index ICH (top panel) and new ICH after diagnosis (bottom panel). Variables are indicator variables unless otherwise noted.
| Outcome: Index ICH | |||||
|---|---|---|---|---|---|
| OR | Lower | Upper | P Value | N | |
| Evidence of old hemorrhage (EOOH) | 3.97 | 2.10 | 7.50 | < 0.001 | 882 |
| Age at diagnosis (decade) | 0.87 | 0.80 | 0.95 | 0.002 | 882 |
| Sex (male) | 0.94 | 0.70 | 1.25 | 0.652 | 882 |
| Deep only venous drainage | 2.51 | 1.66 | 3.79 | < 0.001 | 882 |
| Maximal AVM size (cm) | 0.73 | 0.65 | 0.81 | < 0.001 | 882 |
| Deep location | 1.77 | 1.16 | 2.71 | 0.009 | 882 |
| Associated arterial aneurysm | 1.67 | 1.21 | 2.29 | 0.002 | 882 |
| Outcome: new ICH after diagnosis | |||||
| HR | Lower | Upper | P Value | N | |
| Evidence of old hemorrhage (EOOH) | 3.53 | 1.35 | 9.23 | 0.010 | 643 |
| Age at diagnosis (decade) | 1.19 | 0.92 | 1.53 | 0.178 | 643 |
| Sex (male) | 0.72 | 0.32 | 1.64 | 0.439 | 643 |
| Deep only venous drainage | 0.81 | 0.24 | 2.72 | 0.740 | 643 |
| Maximal AVM size (cm) | 0.91 | 0.68 | 1.21 | 0.510 | 643 |
| Deep location | 1.06 | 0.35 | 3.21 | 0.917 | 643 |
| Associated arterial aneurysm | 1.26 | 0.53 | 2.99 | 0.593 | 643 |
Association of EOOH with Subsequent ICH After Diagnosis
For longitudinal data (n=699), 4.9% of patients experienced a post-diagnosis ICH in 1,626 patient-years of follow-up; there were 34 ICH events: 8 events in 41 patients with EOOH (20%) and 26 events in 658 patients without EOOH (4%).
Cumulative hemorrhage-free survival and number at risk entering each 5-year interval are shown in Figure 2, displayed on a time axis which contains the majority of events. There was a trend for unadjusted EOOH to be associated with a higher risk of subsequent ICH (log-rank test p= .068). Multivariable Cox regression is shown in Table 2. In the multivariable model, EOOH remained an independent predictor of ICH (HR=3.53, 95% CI: 1.35-9.23, p=0.010) after adjusting for our pre-specified ICH risk factors.
Figure 2.
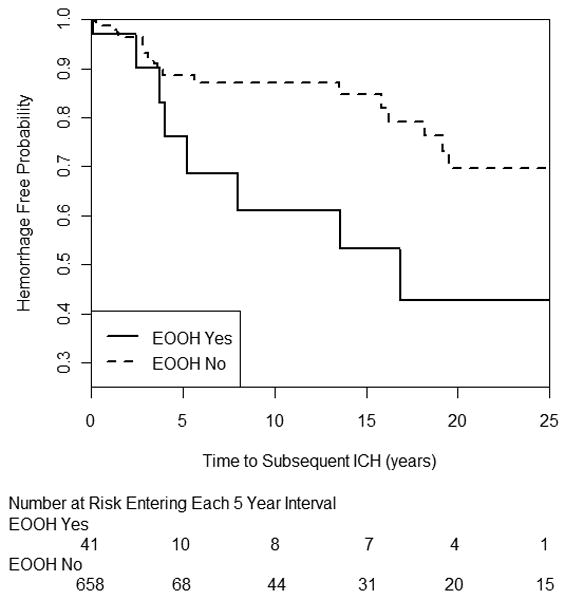
Survival analysis of new ICH after diagnosis by EOOH.
For the mediational analysis, we found that the effect and CIs of index ICH (HR=2.82, 95% CI: 0.99-8.04; p=.052) were similar to that of EOOH (HR=3.53, 95% CI: 1.35-9.23; p=.01) and, more importantly, in a combined model of both index ICH and EOOH, both displayed a 35-56% reduction in HR and became non-significant with no (p=0.464) interaction (data not shown; DNS). These observations are consistent with the two factors being at least partially in the same causal pathway, i.e., previous silent hemorrhage leading to clinical presentation with an ICH representing a similar process that increases risk for ICH subsequent to diagnosis. A multivariable model substituting index ICH for EOOH showed a slighter lower effect for index ICH as the effect for EOOH shown in Table 2 (DNS).
Prevalence of Hemosiderin Positivity and Macrophage Infiltration
A total of 127 patients were included in these analyses. The baseline characteristics are similar to Table 1, the exceptions being the enriched fraction of unruptured cases (70.4%) and a trend to have, as expected, fewer centrally (deep) located lesions, with a lower fraction deep-only venous drainage (DNS).
We explored the relationship of the scoring system as a categorical predictor variable to the index ICH outcome. Although there was a trend toward a relationship between the scored amount of hemosiderin and risk of index ICH, due to the small sample sizes in some cells, the scoring scale was dichotomized into absence and presence for further analysis. The same was done for macrophage infiltration.
The raw categorical variables for macrophage infiltration and hemosiderin were highly correlated (Spearman's rho = .74; p<0.001). Using logistic regression on dichotomized variables, hemosiderin positivity strongly predicted macrophage infiltration (OR=29; 95%CI: 11 – 82; p<.001). Hemosiderin positivity was found in 36.2% (29.6% in unruptured; 47.8% in ruptured; p=.04) and macrophage infiltration was found in 43.3% (37.0% in unruptured and 54.4% in ruptured; p=.058).
Association of Hemosiderin Positivity with Index ICH
Hemosiderin positivity was associated with index ICH in univariate analysis; (OR= 2.18; 95% CI: 1.03—4.61; p=0.042). In multivariable analysis (Table 3; n=79), hemosiderin positivity, age at diagnosis, deep-only venous drainage, and associated arterial aneurysms remained significant (p< 0.05). Full univariate results are provided in Supplemental Table S1 (see http://stroke.ahajournals.org). Excluding cases undergoing pre-surgical embolization or those with remote history of stroke did not affect the relationship of hemosiderin positivity to index ICH in either univariate or multivariable models (DNS). There was no clear relationship between EOOH and hemosiderin positivity, but in the 99 cases with data for both variables, there were only 5 with EOOH, making estimates unreliable (DNS).
Table 3. Multivariable Associations for Association of Hemosiderin Positivity With Index ICH.
| OR | Lower | Upper | P | No. | |
|---|---|---|---|---|---|
| Hemosiderin positive | 3.64 | 1.11 | 12.00 | 0.034 | 79 |
| Age at diagnosis (decade) | 0.58 | 0.42 | 0.79 | 0.001 | 79 |
| Sex, male | 0.66 | 0.22 | 2.02 | 0.470 | 79 |
| Deep only venous drainage | 8.09 | 1.29 | 50.91 | 0.026 | 79 |
| Maximal AVM size, cm | 0.98 | 0.62 | 1.56 | 0.942 | 79 |
| Deep location | 0.36 | 0.05 | 2.75 | 0.326 | 79 |
| Associated arterial aneurysm | 3.25 | 1.24 | 18.90 | 0.023 | 79 |
AVM indicates arteriovenous malformation.
To explore potential confounding by either index ICH or post-diagnostic, pre-operative ICH and surgical harvest, we restricted the logistic regression to include only cases with an interval of less than 4 days between the last clinical hemorrhage and surgical harvest. The OR for hemosiderin positivity remained similar and significant (DNS). We also examined a model that adjusted for the period of time between diagnosis and surgical harvest. The interval between diagnosis and harvest was not significant (p = .561) but the other effect sizes remained similar to those shown in Table 3 (DNS).
We did not undertake time-to-event analyses for new ICH because there were only 5 outcome events in this subset of patients, making the models unstable and the results unreliable.
Association of Hemosiderin Positivity with ex vivo MR Imaging
Supplemental Figures S1 and S2 (see http://stroke.ahajournals.org) show good correspondence between MR susceptibility and histopathological demonstration of iron in resected specimens.
Discussion
We examined two markers of silent intralesional microhemorrhage (SIM) in bAVM patients: (a) EOOH as seen on baseline tomographic imaging; and (b) hemosiderin positivity in resected surgical specimens. We made the following observations: (1) EOOH was independently associated with both index ICH (HR 3.97; p<.001) and new ICH after diagnosis (HR 3.52; p=.006), adjusting for other common ICH risk factors; (2) Hemosiderin positivity was highly prevalent in both unruptured (29.6%) and ruptured (47.8%) bAVM; (3) Hemosiderin positivity was independently associated with index ICH (HR 3.91; p=.03); (4) Hemosiderin positivity and macrophage infiltration were highly correlated (p<0.001); and (5) ex vivo imaging of resected bAVM tissue demonstrated signal loss in areas corresponding to hemosiderin deposition. These data form a solid basis for planning future studies necessary to validate the use of SIM as a risk-stratification tool.
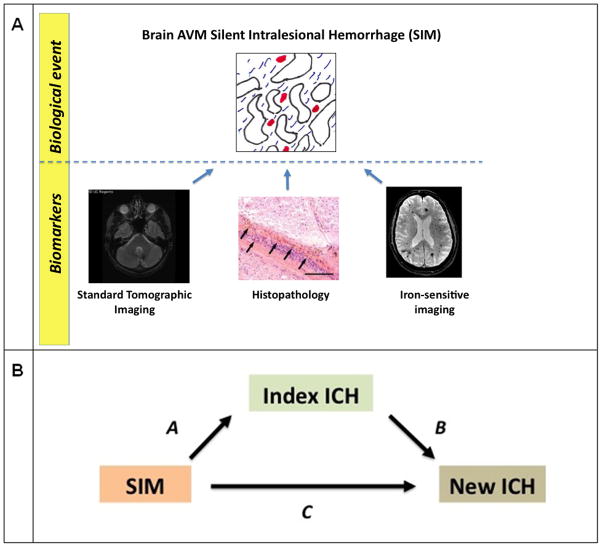
Both hemosiderin positivity and EOOH are markers for the underlying biological phenomenon of SIM (Figure 3). Tissue histopathology is a relatively sensitive and specific means of assessing silent bleeding but is only useful for demonstrating the phenomena and cannot be used practically as a biomarker. The other predictor variable that we used, EOOH, is relatively insensitive because standard clinical imaging was not optimized for detecting iron, especially small amounts. Therefore, neither of these indices is proposed for future development, per se. Rather, they provide evidence that use of modern iron-sensitive imaging is likely to be a fruitful approach.
Figure 3. Conceptual overview.
Panel A: The biological event, Silent Intralesional Microhemorrhage (SIM), can be assessed using biomarkers. In this study, we used standard tomographic imaging (relatively insensitive) and histopathology (impractical), but future studies can leverage newer iron-sensitive MR imaging to develop risk stratification tools.
Panel B: Mediational analysis. EOOH was associated with both Index ICH and new ICH after diagnosis, and appears to lie at least partially in the same causal pathway (arrows A and B). Hemosiderin Positivity was associated with index ICH (arrow A). Future studies with iron-sensitive imaging (ISI) may improve prediction as more sensitive than EOOH and be applicable before treatment.
The presence of prior hemorrhage in BAVMs is by no means a new finding.3-5 Curiously, despite the numerous reports supporting the ontology of the phenomena and suggestions that it be considered in risk evaluation, neither the clinical nor research community has heretofore developed “imaging evidence of prior hemorrhage” as a means of risk stratification. This may be in part attributable to the low sensitivity of older MR techniques. However, there has been considerable progress in susceptibility-weighted imaging.12
It is not clear how SIM corresponds to the imaging construct, “brain microbleeds”, that have garnered considerable recent attention in other cerebrovascular diseases, including the use of microbleeds as a means of risk prediction for recurrent lobar hemorrhages.6, 7 Whatever the relationship to brain microbleeds is, the intralesional microhemorrhage phenotype in bAVM appears to be highly prevalent, and with SWI methodology at higher MR field strength, SIM might represent a useful biomarker for gauging the risk of ICH in patients harboring bAVMs. In addition, the tight coupling of macrophage infiltration with hemosiderin positivity might offer another potential biomarker strategy, using newer methods for imaging macrophages in the brain,13 given the known inflammatory phenotype of the bAVM nidus.14
There are limited studies correlating histological examination with MR evidence of microhemorrhage, but those available suggest a good correspondence.12, 15 Under optimized ex-vivo conditions, we found a good correspondence between histological presence of iron and MR susceptibility in bAVM tissue (see Supplemental Figures 1 and 2, http://stroke.ahajournals.org). However, much more work is needed to systematically compare tissue sections with modern SWI MR techniques.
Our tissue studies suggest an overall prevalence of hemosiderin positivity of ≈40%. We speculate that iron-sensitive SWI imaging will yield at least a similar prevalence. Our tissue sections are but a small sample of the entire bAVM volume. Therefore, despite being sensitive, histological examination may not be specific. Our EOOH variable is the opposite: a large brain volume is interrogated but the MR images that we reviewed to generate the EOOH indicator variable were relatively iron-insensitive, resulting in an EOOH prevalence of only ≈7%. Interestingly, other reports using similar MR methods estimated prevalence of prior hemorrhage as high as 23%.3-5
Although we found that a significant effect of EOOH is associated with new ICH after diagnosis in a time-to-event analysis, in this set of patients we did not find a significant effect of deep-only venous drainage, unlike our previously reported findings and those of others.1, 2 Therefore, the results for the time-to-event analysis should be interpreted with caution.
Our study has important limitations. The data reported here are primarily retrospective in nature and the neuroradiological data collection form was not designed to provide details on EOOH. Except in two cases in which “encephalomacia” was noted, the neuroradiological consultant did not indicate on what the presumption of old hemorrhage was based. Excluding these two cases did not affect our estimates. Although we presume that the majority of the positive responses to the question were due to MR evidence of prior bleeding, we cannot confirm the nature of the findings.
It is possible that our histopathological examination did not discriminate between blood from a SIM and the blood that resulted from an index ICH. An additional and important consideration that applies to the tissue analysis but does not apply to the pre-operative brain imaging studies is that a period of time elapses between bAVM diagnosis and surgical resection. There are two considerations. First, a recent macrohemorrhage might confound interpretation of hemosiderin positivity. The interval between arrival of extravascular blood and its processing into hemosiderin is not precisely known, but based on available information, we chose 4 days to use in our sensitivity analysis.16, 17 Further, it is not known how long hemosiderin remains in the tissue after it is formed and phagocytized by macrophages, although presumably it is a period of many years.
Second, new SIM might occur between diagnosis and surgical harvest, especially with pre-operative embolization. Therefore, hemosiderin positivity may reflect an event that would not be in evidence if, for example, one wished to use iron-sensitive MR to detect SIM for a baseline examination. Assuming that bAVMs are present some period of years before diagnosis, the generation of SIMs should be distributed over the pre-diagnostic natural history, so inclusion of an additional small time interval should minimally influence results. Although we cannot definitively address these points in this retrospective study, our sensitivity analyses suggest that there was not major confounding by timing considerations.
Third, our assessment of EOOH on index imaging in patients with hemorrhage may have introduced bias, in that raters examining imaging with hemorrhage present may be more likely to grade a scan as demonstrating EOOH. A prospective study that considers baseline pre-morbid imaging in unruptured patients can address this concern.
In conclusion, we provide evidence that detection of SIM may represent a biomarker for risk of ICH and would appear to be eminently suited for modern MR imaging to develop a novel risk-stratification tool to further optimize the management of bAVM. In particular, there is a pressing need to improve risk-stratification for unruptured bAVMs: if the nearly completed ARUBA trial (NCT00389181) suggests that even short-term outcome favors non-interventional management, there will be increased demand for additional means to assess ICH risk in the unruptured patient.
Supplementary Material
Acknowledgments
We thank members of the UCSF Brain AVM project (http://avm.ucsf.edu/) for assistance with data collection and report generation.
Sources of Funding: Supported in part by NIH grants R01NS034949 and R01NS027713 (W.L.Y.), P01NS044155 (W.L.Y., H.S.) and K23NS058357 (H.K.).
Appendix
Collaborators
Soonmee Cha, MD, Christopher F. Dowd, MD; Anne Fedoroff, RN, BSN; Elizabeth Gardner, BA; Van V. Halbach, MD; Randall T. Higashida, MD; Philippe Jolivalt, BS; Brad Dispensa, BS, Timothy Shepherd, MD, PhD; Yuanli Zhao, MD, PhD
Footnotes
Presented in part at the 2012 International Stroke Conference.
Disclosures: None
References
- 1.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, et al. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 2.Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 3.Stein BM, Wolpert SM. Arteriovenous malformations of the brain. I: Current concepts and treatment. Arch Neurol. 1980;37:1–5. doi: 10.1001/archneur.1980.00500500031002. [DOI] [PubMed] [Google Scholar]
- 4.Yousem DM, Flamm ES, Grossman RI. Comparison of MR imaging with clinical history in the identification of hemorrhage in patients with cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1989;10:1151–1154. [PMC free article] [PubMed] [Google Scholar]
- 5.Prayer L, Wimberger D, Stiglbauer R, Kramer J, Richling B, Bavinzski G, et al. Haemorrhage in intracerebral arteriovenous malformations: detection with MRI and comparison with clinical history. Neuroradiology. 1993;35:424–427. doi: 10.1007/BF00602821. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordonnier C, Klijn CJ, van Beijnum J, Al-Shahi Salman R. Radiological investigation of spontaneous intracerebral hemorrhage: systematic review and trinational survey. Stroke. 2010;41:685–690. doi: 10.1161/STROKEAHA.109.572495. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Guo Y, Bollen AW, Su H, Young WL. Reduced PDGFR-beta expression after regional Alk1 deletion and VEGF stimulation in the brain is associated with reduced mural cell coverage [Abstract] Stroke. 2012 accepted for presentation at 2012 International Stroke Conference. [Google Scholar]
- 9.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson RP, Awad IA, Batjer HH, Dowd CF, Furlan A, Giannotta SL, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 11.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the rotterdam scan study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 12.Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119:291–302. doi: 10.1007/s00401-009-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiel A, Radlinska BA, Paquette C, Sidel M, Soucy JP, Schirrmacher R, et al. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J Nucl Med. 2010;51:1404–1412. doi: 10.2967/jnumed.110.076612. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62:1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Reuck J, Auger F, Cordonnier C, Deramecourt V, Durieux N, Pasquier F, et al. Comparison of 7.0-T T*-magnetic resonance imaging of cerebral bleeds in post-mortem brain sections of Alzheimer patients with their neuropathological correlates. Cerebrovasc Dis. 2011;31:511–517. doi: 10.1159/000324391. [DOI] [PubMed] [Google Scholar]
- 16.Oehmichen M, Walter T, Meissner C, Friedrich HJ. Time course of cortical hemorrhages after closed traumatic brain injury: statistical analysis of posttraumatic histomorphological alterations. J Neurotrauma. 2003;20:87–103. doi: 10.1089/08977150360517218. [DOI] [PubMed] [Google Scholar]
- 17.Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci. 1995;134(Suppl):102–112. doi: 10.1016/0022-510x(95)00215-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.