Abstract
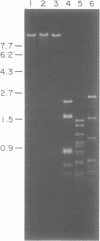
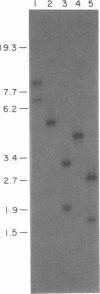
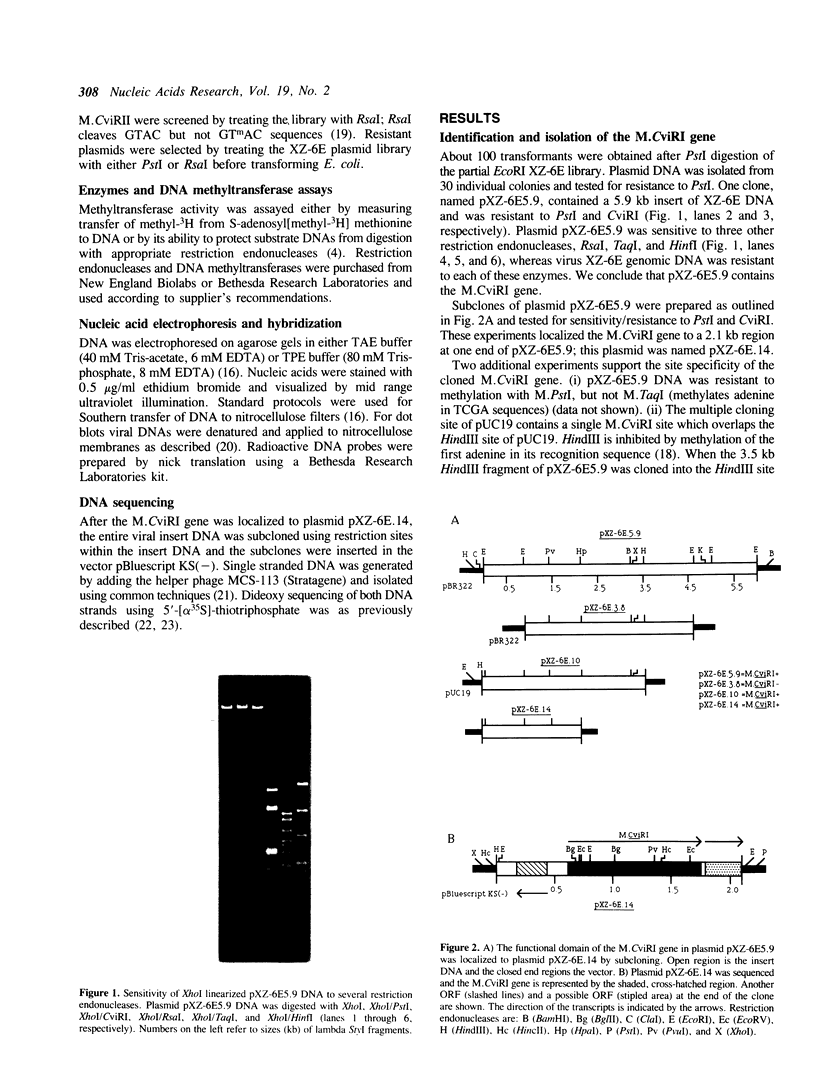
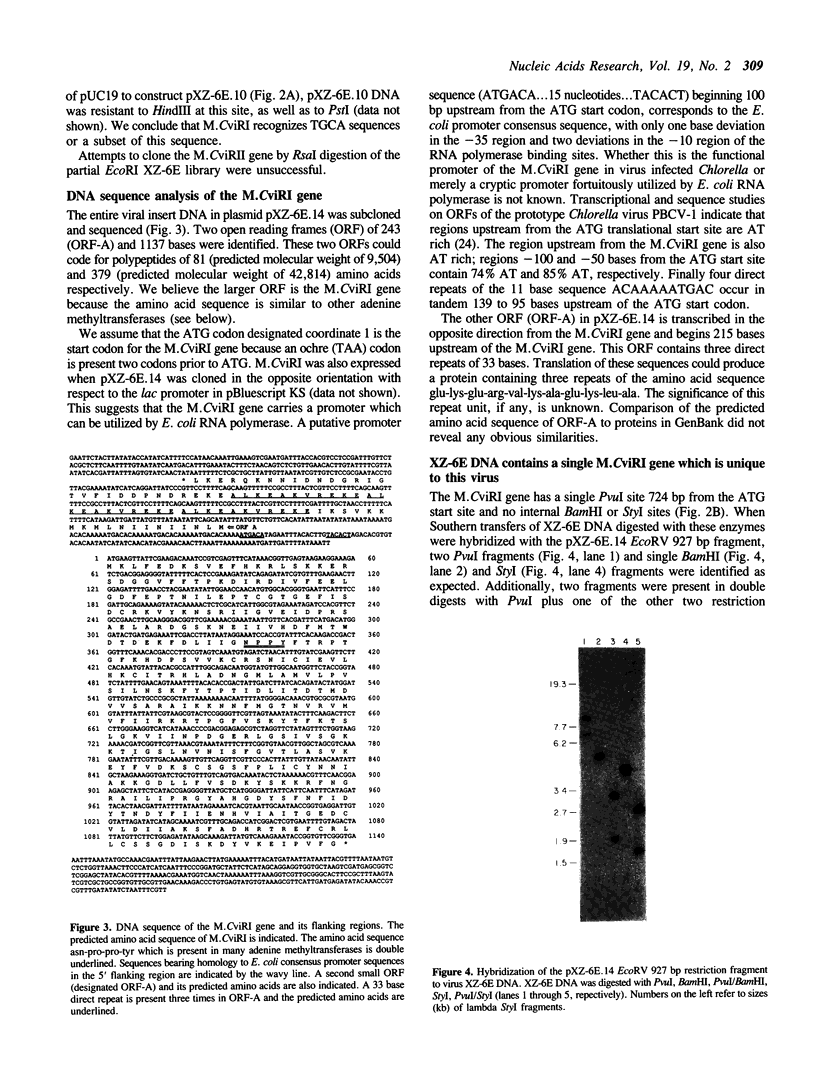
The gene encoding the DNA methyltransferase M.CviRI from Chlorella virus XZ-6E was cloned and expressed in Escherichia coli. M.CviRI methylates adenine in TGCA sequences. DNA containing the M.CviRI gene was sequenced and a single open reading frame of 1137 bp was identified which could code for a polypeptide of 379 amino acids with a predicted molecular weight of 42,814. Comparison of the M.CviRI predicted amino acid sequence with another Chlorella virus and 14 bacterial adenine methyltransferases revealed extensive similarity to the other Chlorella virus enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. E., Blumenthal R. M., Gingeras T. R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983 Feb 11;11(3):837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasegaran S., Lunnen K. D., Smith H. O., Wilson G. G. Cloning and sequencing the HinfI restriction and modification genes. Gene. 1988 Oct 30;70(2):387–392. doi: 10.1016/0378-1119(88)90210-7. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Gupta M., Boyer H. W., Brown W. E., Rosenberg J. M. Sequence analysis of the DNA encoding the Eco RI endonuclease and methylase. J Biol Chem. 1981 Mar 10;256(5):2143–2153. [PubMed] [Google Scholar]
- Hattman S., Wilkinson J., Swinton D., Schlagman S., Macdonald P. M., Mosig G. Common evolutionary origin of the phage T4 dam and host Escherichia coli dam DNA-adenine methyltransferase genes. J Bacteriol. 1985 Nov;164(2):932–937. doi: 10.1128/jb.164.2.932-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszubska W., Aiken C., O'Connor C. D., Gumport R. I. Purification, cloning and sequence analysis of RsrI DNA methyltransferase: lack of homology between two enzymes, RsrI and EcoRI, that methylate the same nucleotide in identical recognition sequences. Nucleic Acids Res. 1989 Dec 25;17(24):10403–10425. doi: 10.1093/nar/17.24.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Kotani H., Sugisaki H., Takanami M. The fokI restriction-modification system. I. Organization and nucleotide sequences of the restriction and modification genes. J Biol Chem. 1989 Apr 5;264(10):5751–5756. [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Mannarelli B. M., Springhorn S. S., Greenberg B. Genetic basis of the complementary DpnI and DpnII restriction systems of S. pneumoniae: an intercellular cassette mechanism. Cell. 1986 Sep 26;46(7):993–1000. doi: 10.1016/0092-8674(86)90698-7. [DOI] [PubMed] [Google Scholar]
- Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989 Mar 20;206(2):313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Daniel A. S., Braymer H. D., Murray N. E. Organization and sequence of the hsd genes of Escherichia coli K-12. J Mol Biol. 1987 Nov 20;198(2):159–170. doi: 10.1016/0022-2836(87)90303-2. [DOI] [PubMed] [Google Scholar]
- Lynn S. P., Cohen L. K., Kaplan S., Gardner J. F. RsaI: a new sequence-specific endonuclease activity from Rhodopseudomonas sphaeroides. J Bacteriol. 1980 May;142(2):380–383. doi: 10.1128/jb.142.2.380-383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Mosig G. Regulation of a new bacteriophage T4 gene, 69, that spans an origin of DNA replication. EMBO J. 1984 Dec 1;3(12):2863–2871. doi: 10.1002/j.1460-2075.1984.tb02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. B., Rao R. N., Smith H. O. Cloning of restriction and modification genes in E. coli: the HbaII system from Haemophilus haemolyticus. Gene. 1978 Apr;3(2):97–112. doi: 10.1016/0378-1119(78)90054-9. [DOI] [PubMed] [Google Scholar]
- Mannarelli B. M., Balganesh T. S., Greenberg B., Springhorn S. S., Lacks S. A. Nucleotide sequence of the Dpn II DNA methylase gene of Streptococcus pneumoniae and its relationship to the dam gene of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4468–4472. doi: 10.1073/pnas.82.13.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Narva K. E., Wendell D. L., Skrdla M. P., Van Etten J. L. Molecular cloning and characterization of the gene encoding the DNA methyltransferase, M.CviBIII, from Chlorella virus NC-1A. Nucleic Acids Res. 1987 Dec 10;15(23):9807–9823. doi: 10.1093/nar/15.23.9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M., McClelland M. The effect of site-specific methylation on restriction-modification enzymes. Nucleic Acids Res. 1987;15 (Suppl):r219–r230. doi: 10.1093/nar/15.suppl.r219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. K., Rubin R. A., Kim S. H., Modrich P. DNA sequences of structural genes for Eco RI DNA restriction and modification enzymes. J Biol Chem. 1981 Mar 10;256(5):2131–2139. [PubMed] [Google Scholar]
- Pósfai J., Bhagwat A. S., Pósfai G., Roberts R. J. Predictive motifs derived from cytosine methyltransferases. Nucleic Acids Res. 1989 Apr 11;17(7):2421–2435. doi: 10.1093/nar/17.7.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Trimarchi R., Revel H. Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12. Genetics. 1989 Jun;122(2):279–296. doi: 10.1093/genetics/122.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. M., Woodley K., Baird M. Quantitative autoradiography of dot blots using a microwell densitometer. Biotechniques. 1989 Jul-Aug;7(7):680–688. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Burbank D. E., Meister B., Skrdla M. P., Meints R. H., Hattman S., Swinton D., Van Etten J. L. Characterization of viruses infecting a eukaryotic Chlorella-like green alga. Virology. 1986 Apr 15;150(1):170–177. doi: 10.1016/0042-6822(86)90276-x. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Graves M., Korth K., Ziegelbein M., Brumbaugh J., Grone D., Meints R. H. Transcription and sequence studies of a 4.3-kbp fragment from a ds-DNA eukaryotic algal virus. Virology. 1990 Jun;176(2):515–523. doi: 10.1016/0042-6822(90)90021-i. [DOI] [PubMed] [Google Scholar]
- Shields S. L., Burbank D. E., Grabherr R., van Etten J. L. Cloning and sequencing the cytosine methyltransferase gene M. CviJI from Chlorella virus IL-3A. Virology. 1990 May;176(1):16–24. doi: 10.1016/0042-6822(90)90225-g. [DOI] [PubMed] [Google Scholar]
- Slatko B. E., Benner J. S., Jager-Quinton T., Moran L. S., Simcox T. G., Van Cott E. M., Wilson G. G. Cloning, sequencing and expression of the Taq I restriction-modification system. Nucleic Acids Res. 1987 Dec 10;15(23):9781–9796. doi: 10.1093/nar/15.23.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson F. H., Greene P. J. Nucleotide sequence of the gene encoding the RsrI methyltransferase. Nucleic Acids Res. 1989 Dec 25;17(24):10503–10503. doi: 10.1093/nar/17.24.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault G., Roy P. H., Howard K. A., Benner J. S., Brooks J. E., Waters A. F., Gingeras T. R. Nucleotide sequence of the PaeR7 restriction/modification system and partial characterization of its protein products. Nucleic Acids Res. 1985 Dec 9;13(23):8441–8461. doi: 10.1093/nar/13.23.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Schuster A. M., Meints R. H. Lytic viruses infecting a Chlorella-like alga. Virology. 1985 Jan 15;140(1):135–143. doi: 10.1016/0042-6822(85)90452-0. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A., Donelson J. E. The organization and complete nucleotide sequence of the PstI restriction-modification system. J Biol Chem. 1984 Jun 25;259(12):8015–8026. [PubMed] [Google Scholar]
- Xia Y. N., Burbank D. E., Uher L., Rabussay D., Van Etten J. L. IL-3A virus infection of a Chlorella-like green alga induces a DNA restriction endonuclease with novel sequence specificity. Nucleic Acids Res. 1987 Aug 11;15(15):6075–6090. doi: 10.1093/nar/15.15.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Burbank D. E., Uher L., Rabussay D., Van Etten J. L. Restriction endonuclease activity induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1430–1439. doi: 10.1128/mcb.6.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Morgan R., Schildkraut I., Van Etten J. L. A site-specific single strand endonuclease activity induced by NYs-1 virus infection of a Chlorella-like green alga. Nucleic Acids Res. 1988 Oct 25;16(20):9477–9487. doi: 10.1093/nar/16.20.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Narva K. E., Van Etten J. L. The cleavage site of the RsaI isoschizomer, CviII, is G decreases TAC. Nucleic Acids Res. 1987 Dec 10;15(23):10063–10063. doi: 10.1093/nar/15.23.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Van Etten J. L. DNA methyltransferase induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1440–1445. doi: 10.1128/mcb.6.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Burbank D. E., Van Etten J. L. Restriction endonuclease activity induced by NC-1A virus infection of a Chlorella-like green alga. Nucleic Acids Res. 1986 Aug 11;14(15):6017–6030. doi: 10.1093/nar/14.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. P., Burbank D. E., Van Etten J. L. Chlorella viruses isolated in China. Appl Environ Microbiol. 1988 Sep;54(9):2170–2173. doi: 10.1128/aem.54.9.2170-2173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]