Abstract
Marijuana dependence is a substantial public health problem, with existing treatments showing limited efficacy. In laboratory and clinical studies, the cannabinoid receptor 1 (CB1) agonist oral Δ9tetrahydrocannabinol (THC; dronabinol) has been shown to decrease marijuana withdrawal, but not relapse. Dronabinol has poor bioavailability, potentially contributing to its failure to decrease relapse. The synthetic THC analogue, nabilone, has better bioavailability than dronabinol. We therefore aimed to characterize nabilone's behavioral and physiological effects across a range of acute doses in current marijuana smokers, and compare these with dronabinol's effects. Participants (4F; 10M) smoking marijuana 6.6 (SD = 0.7) days/week completed this outpatient, within-subjects, double-blind, randomized protocol. Over 7 sessions, the time-dependent subjective, cognitive, and cardiovascular effects of nabilone (2, 4, 6, 8 mg), dronabinol (10, 20 mg) and placebo were assessed. Nabilone (4, 6, 8 mg) and dronabinol (10, 20 mg) increased ratings of feeling a good effect, a strong effect, and/or `high' relative to placebo; nabilone had a slower onset of peak subjective effects than dronabinol. Nabilone (6, 8 mg) modestly lowered psychomotor speed relative to placebo and dronabinol. There were dose-dependent increases in heart rate after nabilone, and nabilone (2 mg) and dronabinol (10 mg) decreased systolic blood pressure. Thus, nabilone produced sustained, dose-related increases in positive mood, few cognitive decrements, and lawful cardiovascular alterations. It had a longer time to peak effects than dronabinol and effects were more dose-related, suggesting improved bioavailability. Nabilone was well-tolerated by marijuana smokers, supporting further testing as a potential medication for marijuana dependence.
Keywords: agonist treatment, dose-effect profile, dronabinol, marijuana dependence, nabilone
INTRODUCTION
Marijuana is the most commonly used illegal drug in the United States (SAMSHA 2010) and internationally (United Nations Office on Drugs and Crime 2007). Although most people who initiate marijuana use do not develop problematic use of the drug (Brook et al. 2011), a subset progress to smoking marijuana daily (around 8% of past year users; Chen et al. 1997). A sizable minority (some 20%) of daily or near daily marijuana smokers meet criteria for marijuana dependence (Chen et al. 1997). Moreover, upon cessation of daily marijuana use a characteristic constellation of withdrawal symptoms including craving, anxiety, irritability, and sleep and appetite disruptions, has been demonstrated to occur in both laboratory (Haney et al. 2008; Haney et al. 2004; Haney et al. 1999b) and clinical (Vandrey et al. 2008) settings. Thus, although the proportion of marijuana users who experience dependence is small, the high prevalence of marijuana smoking means that the absolute number of individuals with problematic use of the drug is greater than that for illegal drugs with higher abuse liability (Budney et al. 2007a; SAMSHA 2010).
Consistent with this, estimates indicate that some 1.2 million people in the United States received treatment for marijuana use in 2009 (SAMSHA 2010). Existing treatments are primarily behavioral and psychotherapeutic, and have been reported to assist in reducing marijuana use and increasing cessation rates (Budney et al. 2006; Kadden et al. 2007; MTP Research Group 2004). However, as is the case for other drugs, relapse rates remain high. For example, one study assessing the efficacy of voucher-based contingency management, alone and combined with cognitive behavioural therapy (CBT), reported that only 17% (vouchers alone) to 37% (vouchers plus CBT) of patients were abstinent 12 months after treatment cessation (Budney et al. 2006). To better address the needs of treatment-seeking marijuana smokers it is clear that new treatments and adjuncts to existing treatments are needed. Combinations of psychotherapy and pharmacotherapy are most effective in treatment of primary psychiatric conditions such as depression (Zobel et al. 2011) and such strategies enhance treatment for other drugs such as cigarettes (Grassi et al. 2011) and heroin (Nunes et al. 2006), suggesting that a similar approach may improve treatment of marijuana dependence. However, there is, as yet, no FDA-approved medication to aid in cessation or reduction of marijuana use.
One approach successfully employed in treating opiate and tobacco dependence is the use of agonist medications to alleviate withdrawal symptoms that may precipitate relapse (Stapleton and Sutherland 2011; Threlkeld et al. 2006). The cannabinoid receptor type 1 and 2 (CB1 and CB2) agonist dronabinol (synthetic Δ9tetrahydrocannabinol [THC]; Marinol®) reduces withdrawal symptoms in abstinent marijuana smokers (Budney et al. 2007b; Haney et al. 2004; Levin et al. 2011), however dronabinol alone did not affect relapse rates in laboratory (Haney et al. 2008) or clinical (Levin et al. 2011) studies. Dronabinol has low bioavailability (from 4–20%; Ben Amar 2006; McGilveray 2005), and its effects do not reliably show clear dose dependency (e.g. see Haney et al. 1999a), perhaps contributing to its lack of effect on marijuana relapse. A substantial further limitation is that urinary metabolites of dronabinol are identical to those of marijuana (Lile et al. 2010), preventing differentiation between compliance with medication and ongoing marijuana use in clinical and research settings.
An alternative to dronabinol is nabilone (Cesamet®), a synthetic cannabinoid analogue of THC that is FDA-approved for nausea and emesis in chemotherapy patients. Nabilone produces urinary metabolites distinct from those of marijuana (Fraser and Meatherall 1989). In addition, nabilone is more potent than dronabinol, has greater bioavailability (at least 60%; Ben Amar 2006; Lemberger et al. 1982), and demonstrates dose linearity (Lemberger et al. 1982). Two recent drug discrimination studies have tested a range of dronabinol and nabilone doses in a marijuana-using sample. One study (N=6) showed that nabilone (2, 3 and 5 mg) and dronabinol (10, 15 and 25 mg) substituted for the test dose of dronabinol (25 mg), indicating substantial overlap in the interoceptive properties of the two drugs (Lile et al. 2010). Dronabinol and nabilone also produced lawful increases in marijuana-like subjective effects such as feeling a good drug effect, feeling `high', and feeling `stoned', as well as increasing heart rate. The second study (N=6) demonstrated that nabilone (1, 3 mg) pretreatment enhanced the discriminative-stimulus effects of dronabinol (5, 10, 15 mg), demonstrating the safety of combining these two orally-administered cannabinoid agonists (Lile et al. 2011).
The purpose of the present study was to compare the dose-effect profile and time course of the effects of nabilone and dronabinol in volunteers who smoke marijuana heavily (repeatedly each day, 6–7 days/week), as this is the most relevant population in which to study potential treatment approaches for marijuana dependence (Haney 2008). Because regular marijuana smokers are tolerant to cannabinoid effects (Haney 2007; Hart et al. 2001), we administered higher nabilone doses than have been previously tested. Characterizing the effects of a range of doses of nabilone relative to dronabinol on: 1) self-reported mood state; 2) cognitive function; and 3) cardiovascular function will provide essential information about appropriate doses to employ to assess the effects of nabilone on marijuana withdrawal and relapse.
MATERIALS AND METHODS
Participants
Healthy male and female regular marijuana smokers were recruited using newspaper and Internet advertisements. Participants were required to 1) be between 21 and 45 years of age; 2) state that they were not seeking treatment for marijuana use; and 3) agree to use effective birth control for the duration of the study (females). Exclusion was based on: 1) Axis 1 psychiatric disorder requiring medical intervention (DSM-IV criteria; APA 1994); 2) inability to abstain from drugs other than marijuana for the study duration, verified by urine toxicology; 3) medical findings counterindicating participation; 4) inability to perform study procedures; and 5) current pregnancy or lactation (female candidates). Prior to enrolment, candidates underwent comprehensive medical and psychiatric screening including electrocardiogram, urine and blood toxicology, and clinical interview. At enrolment, participants provided written informed consent. At study completion, they underwent full debriefing and received compensation for participation. All study procedures were approved by the New York State Psychiatric Institute's (NYSPI) Institutional Review Board and were in accordance with the Helsinki Declaration of 1964, revised in 2008.
Experimental Protocol
This outpatient study employed a within-subjects, randomized, double-blind design. Participants initially underwent a practice session in which no medication was administered to become familiar with study procedures. They then completed seven, 6-hour drug-administration sessions separated by at least 48 hours to allow for study medication clearance. Sessions took place in a comfortable laboratory environment; participants were tested individually to prevent effects of social interaction with other participants on responses to the study medications. Participants were instructed to not eat breakfast on the morning of sessions, and to not consume alcohol for 24 hours prior to session initiation. A breathalyzer test upon arrival confirmed compliance with this requirement; participants also provided samples for urine toxicology screens to ensure no recent use of drugs other than marijuana. Participants were required to abstain from smoking marijuana or cigarettes from midnight the night prior to sessions. To confirm compliance, breath carbon monoxide was tested and was required to be ≤ 8ppm prior to commencement of the session. Female participants' urine was also screened for pregnancy twice during participation, which was approximately 3 weeks in duration. After these tests, participants consumed a light breakfast of their choice that was standardized across sessions. Baseline subjective, cognitive, and cardiovascular measurements (see below) were collected. Study medications were administered around 60 minutes after arrival. Subjective, cognitive and cardiovascular measurements were collected at 30-minute intervals for two hours following medication administration; thereafter measurements were taken hourly for three hours. Cigarette-smoking participants were allowed to smoke cigarettes at the same time in each session to prevent the onset of nicotine withdrawal over the 6-hour session (Parrott et al. 1996). Following final assessments, participants were required to pass a standardized field sobriety test prior to leaving the laboratory. They were instructed not to drive a car for 8 hours following sessions and were reimbursed for the cost of public transportation to and from each session.
Over the seven sessions, participants received nabilone (2, 4, 6, 8 mg), dronabinol (10, 20 mg), and placebo in randomized order. Nabilone (0, 1 mg; Meda Pharmaceuticals Inc, Somerset, NJ) and dronabinol (10 mg; Unimed Pharmaceuticals, Buffalo Grove, IL) capsules were overencapsulated by the NYSPI Pharmacy in size 00 capsules with lactose filler. Because one condition (nabilone 8 mg) required that a total of 8 capsules (1 mg nabilone each) be administered, 8 capsules were administered in each session with the number of placebo capsules varying according to active condition. The lowest nabilone dose selected is equivalent to the upper limit of the recommended dose for nausea; doses higher than this (4, 6, and 8 mg) were also employed because the regular marijuana smokers recruited were expected to show tolerance to cannabinoid effects (Haney 2007; Hart et al. 2001). We have previously shown doses of 10 and 20 mg of dronabinol to be well-tolerated in marijuana smokers (Hart et al. 2005), and repeated doses of 10 and 20 mg dronabinol alleviate withdrawal symptoms in abstinent marijuana smokers (Budney et al. 2007b; Haney et al. 2004; Levin et al. 2011).
Outcome Measures
Subjective Mood and Drug Effect
Subjective effects of the capsules were measured using a series of Visual Analogues Scales (VAS; Folstein and Luria 1973) and a Capsule Ratings Form (CRF; Haney 2007) that we have previously found to be sensitive to the effects of oral cannabinoids (Bedi et al. 2010; Haney 2007; Haney et al. 2008). The VAS consisted of 44 descriptors assessing mood (e.g. `content'), medication effects (e.g. `bad effect'), and physical symptoms (e.g. `blurred vision'). At each assessment, participants were required to rate the extent to which each descriptor applied to them at that time on a 100mm computer-presented visual analogue scale anchored with `not at all' and `extremely'. To limit the number of analyses conducted, we reduced 35 of the 44 items into 7 cluster scales, calculated as arithmetic means of individual items based on a previously conducted factor analysis (see Haney 2007). The seven clusters employed were 1) High (consisting of the descriptors `good drug effect,' and `high'); 2) Miserable (`depressed,' `irritable,' and `miserable'); 3) Tired (`clumsy,' `sedated,' `sleepy,' `withdrawn,' `unmotivated,' and `tired'); 4) On Edge (`anxious,' `jittery,' `restless,' `stimulated,' and `on edge'); 5) Bad Effect (`blurred vision,' `chills,' `dizzy,' `headache,' `heart pound,' `nauseous,' `stomach pain,' `upset stomach,' and `bad drug effect'); 6) Content (`alert', `energetic', `mellow', `self-confident', `social', `talkative', and `content'); and 7) Confused (`can't concentrate', `forgetful', and `confused'). In addition to these cluster scales, we also included two individual items that were not included in the clusters: `hunger' and `marijuana craving'.
The CRF, presented in paper and pencil format, consisted of 5 items assessing the extent to which participants: felt a good drug effect, felt a bad drug effect, felt a strong effect, liked the effect they felt, and would be willing to take the drug again. Each item was rated on a 100 mm visual analogue scale.
Cognition
Cognitive function was assessed with a computerized performance battery including: a Digit Span immediate and delayed recall and recognition task (DIG; 2 minutes); a Divided Attention Task (DAT; 10 minutes); a Digit-Symbol Substitution Task (DSST; 3 minutes); and a Repeated Acquisition Task (RAT; 3 minutes). This battery, designed for repeated assessments, measures basic as well as higher-level cognitive functions including psychomotor speed, simple and divided attention, and verbal and working memory. We have previously shown this battery to be sensitive to the effects of oral cannabinoids (Bedi et al. 2010; Hart et al. 2005).
Cardiovascular Function
Heart rate and seated blood pressure were measured with a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa CA). In addition, because nabilone can produce orthostatic hypotension (Lemberger et al. 1982), we repeatedly assessed change in systolic and diastolic blood pressure from sitting to standing during each session; no participant was allowed to leave a session until vital signs, including orthostatic blood pressure, were normal.
Data Analyses
The outcome variables for subjective (VAS and CRF scores), cognitive (DSST, DAT, DIG, and RAT scores) and physiological (heart rate, blood pressure) measures were calculated as the peak score of the 6 (cognitive tests) or 7 (subjective and cardiovascular assessments) post-baseline measurements taken over each session. Prior to main analyses, we assessed for differences in baseline scores across sessions for all variables except the CRF, which was not employed at baseline because no capsule had been administered; no significant differences were observed. Effects of drug condition were assessed with single-factor repeated measures Analyses of Variance (ANOVAs) followed by planned contrasts comparing: 1) nabilone 2, 4, 6 and 8 mg with placebo; 2) dronabinol 10 and 20 mg with placebo; 3) nabilone 2, 4, 6, and 8 mg with dronabinol 10 mg; and 4) nabilone 2, 4, 6, and 8 mg with dronabinol 20 mg. With this approach we aimed to assess both the effects of the active drug conditions relative to placebo, and to compare the nabilone and dronabinol conditions. Alpha was set at p < 0.05. Where no significant departure from sphericity was indicated by Mauchley's test of sphericity (p > 0.05), planned contrasts were calculated using the omnibus ANOVA residual mean squares and degrees of freedom (see Sheskin 2000). In the case that a significant departure from sphericity was observed, we conservatively employed the individual contrast error term (Keppel 1991). We observed isolated univariate outliers (z > ±3.29) across measures. In all except two cases, removal of the outlier did not affect outcomes and original data were retained. In two outcome measures (VAS Confused cluster and CRF Bad Drug Effect) removal of the outlying data point altered the results; therefore results are presented with data from one participant removed. Analyses were undertaken using SPSS 18.0 (SPSS, Chicago, Illinois).
RESULTS
Fourteen participants (4 female; 10 male) completed the study. A further five volunteers (3 female; 2 male) started but did not complete the protocol. Of these, one dropped from the study reporting that she disliked the medication effects, one reportedly felt uncomfortable in the laboratory environment, one was dropped after twice producing urine samples positive for benzodiazepines, and two did not attend sessions as scheduled.
Of participants who completed the study, four identified as White (one was Hispanic), nine were Black (three were Hispanic), and one was reportedly of mixed racial origin. They had a mean age of 27.9 (SD = 5.1) years and reported an average of 13.1 (SD = 2.5) years of education. Participants reportedly smoked marijuana 6.6 (SD = 0.7) days per week, smoking 6.9 (SD = 4.5) `joints' (marijuana cigarettes) per day at a weekly cost of $USD80.50 (SD = 59.8). On average, they reported having first smoked marijuana at 13.9 (SD = 3.5) years of age and had smoked the drug regularly for 9.9 (SD = 6.7) years. Twelve reported some current alcohol consumption, drinking on average 2.8 (SD = 1.3) alcoholic drinks on 1.2 (SD = 1.2) days per week. Nine were current cigarette smokers, smoking 4.4 (SD = 3.2) cigarettes per day.
Subjective Drug Effects and Mood State
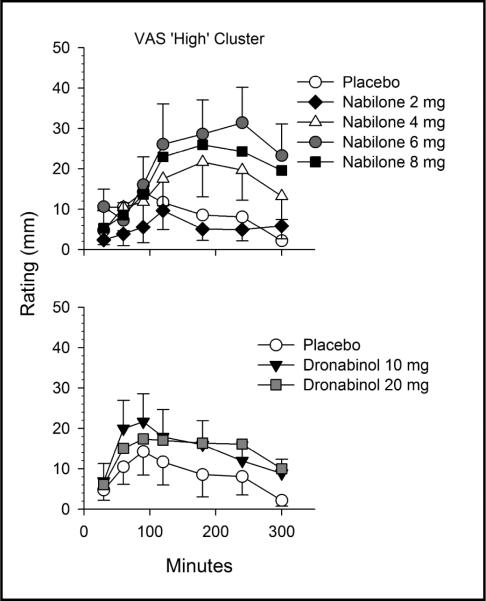
The higher doses of nabilone were rated as producing positive drug effects relative to placebo. As shown in Figure 1, nabilone (6, 8 mg) increased CRF `good drug effect' relative to placebo, whereas the lowest dose of nabilone (2 mg) produced lower peak ratings on this measure compared to dronabinol (10 mg). Figure 2 shows a similar pattern for the VAS `high' cluster; the highest nabilone doses (6, 8 mg) increased ratings compared to placebo; nabilone (6 mg) also produced higher ratings relative to dronabinol (20 mg). On both the CRF `good drug effect' and VAS `high' clusters, the intermediate dose of nabilone (6 mg) produced the highest ratings at 39.7/100 ± 10.8 and 39.5/100 ± 10.5 respectively, revealing an inverted u-shaped function.
Figure 1.
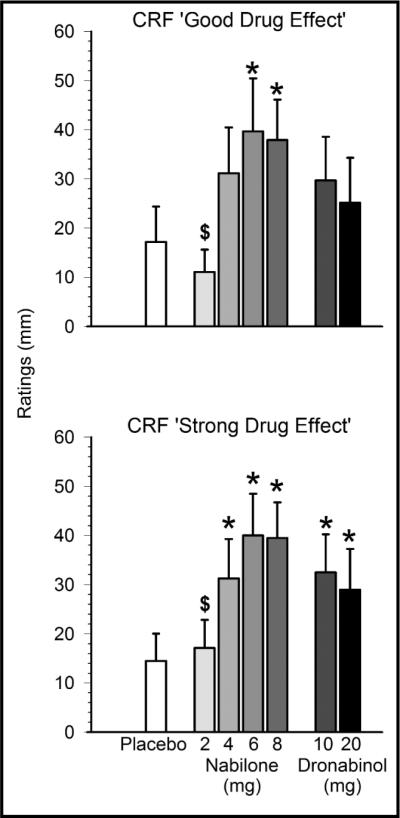
Effects of nabilone (2, 4, 6, 8 mg) and dronabinol (10, 20 mg) on self-rated good drug effect (top) and strong drug effect (bottom) in regular marijuana smokers. Data are means of peak scores from seven assessments taken after capsule administration (± SEM; maximum possible score = 100). Asterisks denote a significant difference from placebo (p < 0.05) in the indicated condition. $ denotes a significant difference from dronabinol (10 mg). CRF = Capsule Rating Form.
Figure 2.
Time course of effects of nabilone (2, 4, 6, 8 mg; top) and dronabinol (10, 20 mg; bottom) on self-rated feelings of being high in regular marijuana smokers. Data are means (± SEM; maximum possible score = 100). VAS = Visual Analogue Scale.
On the CRF `strong drug effect' measure (Figure 1), the three higher doses of nabilone (4, 6, 8 mg) as well as both doses of dronabinol increased scores compared to placebo. Nabilone (2 mg) produced lower ratings on `strong drug effect' than dronabinol (10 mg). The highest doses of nabilone (6, 8 mg) significantly increased CRF ratings of drug likeability and willingness to take the drug again relative to placebo. Nabilone (8 mg) also increased ratings of willingness to take the drug again compared to dronabinol (20 mg), with no other differences observed between nabilone and dronabinol on these measures (see Table 1).
Table 1.
Effects of Nabilone and Dronabinol on Peak Scores for Subjective Mood State and Cognitive Function
| PBO Meana (SEM) |
NAB2 Meana (SEM) |
NAB4 Meana (SEM) |
NAB6 Meana (SEM) |
NAB8 Meana (SEM) |
DRON10 Meana (SEM) |
DRON20 Meana (SEM) |
Significant Planned Comparisons* |
|
|---|---|---|---|---|---|---|---|---|
| CRF Capsule | 21.7 | 17.0 | 32.8 | 38.5 | 38.5 | 29.8 | 28.4 | NAB6 > PBO |
| Likingb | (9.7) | (8.2) | (9.7) | (10.0) | (8.6) | (8.8) | (10.4) | NAB8 > PBO |
| CRF Would Take | 24.1 | 15.2 | 30.3 | 40.5 | 34.1 | 29.3 | 26.5 | NAB6 > PBO |
| Capsule Againb | (9.4) | (8.1) | (10.3) | (11.3) | (9.9) | (9.8) | (9.9) | NAB8 > PBO |
| NAB8 > DRON20 | ||||||||
| VAS Tiredb | 10.1 | 8.8 | 11.5 | 16.1 | 13.0 | 6.8 | 12.0 | NAB6 > PBO |
| (2.9) | (1.9) | (2.8) | (4.1) | (2.5) | (1.7) | (3.9) | NAB6 > DRON10 | |
| NAB8 > DRON10 | ||||||||
| VAS On Edgeb | 9.0 | 8.4 | 9.2 | 12.2 | 10.4 | 6.3 | 7.3 | NAB6 > DRON10 |
| (2.9) | (3.2) | (2.2) | (3.5) | (3.3) | (2.4) | (3.2) | NAB8 > DRON10 | |
| NAB6 > DRON20 | ||||||||
| VAS Marijuana | 63.6 | 58.6 | 64.8 | 63.9 | 64.9 | 64.9 | 55.5 | - |
| Cravingb,c | (8.5) | (7.7) | (9.9) | (9.3) | (9.4) | (9.4) | (11.2) | |
| DAT Number of | 9.3 | 5.9 | 6.5 | 6.2 | 4.5 | 7.3 | 4.0 | DRON10 > NAB8 |
| False Alarms | (4.1) | (2.3) | (2.3) | (1.5) | (1.3) | (2.0) | (1.4) | |
| DAT Total | 19692.4 | 15414.1 | 14195.1 | 16172.5 | 18696.2 | 16064.9 | 18549.5 | PBO > NAB2 |
| Tracking Distance | (4721.1) | (3480.3) | (2082.1) | (3569.4) | 3125.1 | (3618.3) | (4489.3) | PBO > DRON10 |
| DSST Total | 91.2 | 88.4 | 87.0 | 81.6 | 85.4 | 89.6 | 88.4 | PBO > NAB6 |
| Correct | (3.8) | (3.3) | (2.8) | (3.6) | (2.6) | (3.6) | (3.3) | PBO > NAB8 |
| DRON10 > NAB6 | ||||||||
| DRON20 > NAB6 | ||||||||
| DSST % Correct | 98.7 | 98.4 | 98.2 | 97.8 | 98.1 | 98.2 | 98.9 | PBO > NAB6 |
| (0.5) | (0.4) | (0.8) | (0.6) | (0.9) | (0.6) | (0.4) | PBO > NAB8 | |
| DRON20 > NAB4 | ||||||||
| DRON20 > NAB6 | ||||||||
| DRON20 > NAB8 | ||||||||
| RAT Number of | 57.2 | 55.1 | 50.9 | 48.9 | 53.0 | 54.7 | 50.3 | PBO > NAB4 |
| Errors | (5.5) | (4.2) | (4.8) | (4.7) | (5.2) | (5.9) | (4.7) | PBO > NAB6 |
| PBO > DRON20 |
PBO = placebo; NAB2 = nabilone 2 mg; NAB4 = nabilone 4 mg; NAB6 = nabilone 6 mg; NAB8 = nabilone 8 mg; DRON10 = dronabinol 10 mg; DRON20 = dronabinol 20 mg; SEM = standard error of the mean; CRF = capsule rating form; VAS = visual analogue scale; DAT = divided attention task; DSST = digit symbol substitution task; RAT = rapid acquisition task.
p < 0.05
Individual subject peak scores across the seven (or six for cognitive measures) time points after capsule administration were calculated; data are means of these peak scores.
Scores range from 0 to 100.
N = 13 due to missing data.
Nabilone (6 mg) increased ratings on the VAS `tired' cluster relative to placebo, whereas both higher doses of nabilone (6, 8 mg) increased `tired' ratings compared to the lower dose of dronabinol (10 mg). Nabilone (6, 8 mg) increased ratings on the VAS `on edge' cluster compared to dronabinol (10 mg); nabilone (6 mg) also increased scores on this measure relative to dronabinol (20 mg). However, ratings of `tired' and `on edge' were low with no drug condition producing peak scores higher than 17 out of a possible 100 on either of these measures (see Table 1).
There were no adverse reactions requiring medical intervention observed during the study and no effect of drug condition on the majority of negative affect and physical symptom measures (other than small changes in `tired' and `on edge'). Neither nabilone nor dronabinol affected ratings of `bad drug effects', or feeling `miserable', `confused', `content', or `hungry' (data not presented). There was also no effect of medication on VAS ratings of craving for marijuana (see Table 1).
As shown in Figure 2, nabilone had a longer time to peak subjective effects than did dronabinol. Peak effects on VAS `high' ratings were recorded at 120 minutes for nabilone (2 mg), 180 minutes for both the 4 and 8 mg doses, and 240 minutes post-administration for nabilone (6 mg). Conversely, peak effects for both doses of dronabinol occurred 90 minutes after capsule administration, consistent with earlier findings (e.g. Haney et al. 2005). A similar pattern, of nabilone producing later peak subjective effects than dronabinol, was observed on other measures such as CRF `strong drug effect'.
Cognitive Function
There were limited effects of nabilone on the range of cognitive functions assessed. As shown in Figure 3, nabilone reduced psychomotor speed relative to placebo and dronabinol, with nabilone (6, 8 mg) decreasing the total number of items attempted on the Digit Symbol Substitution Task (DSST) compared to placebo and dronabinol (10 mg), and nabilone (6 mg) decreasing the number attempted compared to dronabinol (20 mg). Nabilone (6, 8 mg) also decreased the number of items correct on the DSST compared to placebo; nabilone (6 mg) decreased DSST scores compared to both doses of dronabinol (Table 1). The higher doses of nabilone (6, 8 mg) decreased the percentage correct on the DSST relative to placebo and dronabinol (20 mg); nabilone 4 mg decreased DSST percent correct compared to dronabinol only (20 mg; see Table 1).
Figure 3.
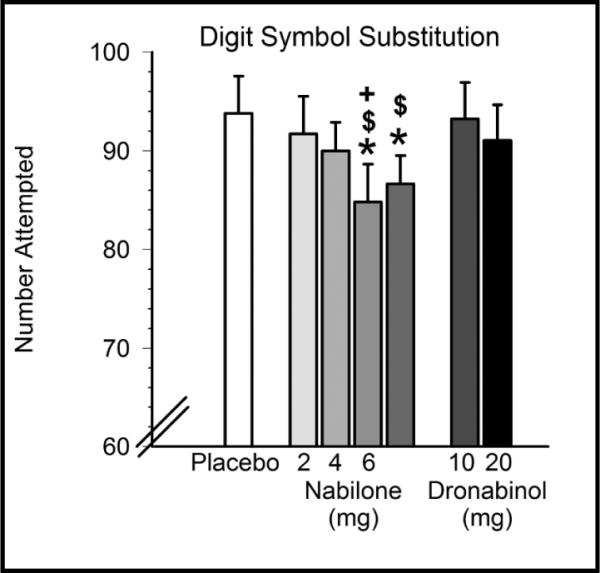
Effects of nabilone (2, 4, 6, 8 mg) and dronabinol (10, 20 mg) on psychomotor speed in regular marijuana smokers. Data are means of peak scores from six assessments taken after capsule administration (± SEM). Asterisks denote a significant difference from placebo (p < 0.05) in the indicated condition. $ denotes a significant difference from dronabinol (10 mg). + denotes a significant difference from dronabinol (20 mg).
The number of false alarms on the Divided Attention Task (DAT) was higher after dronabinol administration (10 mg) than nabilone (8 mg; Table 1) and the total tracking distance on the DAT was lower after nabilone (2 mg) and dronabinol (10 mg) than placebo (see Table 1). There was no effect of medication condition on the number of hits, hit latency, or maximum tracking speed on the DAT, the number of items entered in the Digit Span task, or on immediate or delayed recall or recognition scores (data not presented).
On the Repeated Acquisition Task (RAT), which taps, among other things, working memory, there was no effect of medication on the total number of trials completed (data not presented). Indeed, the number of errors made in this task was higher on placebo relative to nabilone (4, 6 mg) and dronabinol (20 mg; Table 1).
Cardiovascular Function
As shown in Figure 4, nabilone produced dose-dependent increases in heart rate: heart rate following nabilone administration (4, 6, 8 mg) was significantly higher than when placebo or dronabinol (10, 20 mg) were given. Nabilone (2 mg) and dronabinol (10 mg) resulted in decreases in seated systolic blood pressure relative to placebo. There was no effect of medication condition on seated diastolic blood pressure (data not presented). Of 686 measurements of standing versus seated blood pressure taken after drug administration, 11 met criteria for orthostatic hypotension (>20 mmHg decrease in systolic blood pressure and/or >10 mmHg decrease in diastolic blood pressure upon standing). Of these measurements, 2 occurred after placebo administration, one after nabilone (4 mg), 2 after nabilone (6 mg), 4 after nabilone (8 mg), and 2 occurred after administration of dronabinol (20 mg). None of these instances required medical intervention.
Figure 4.
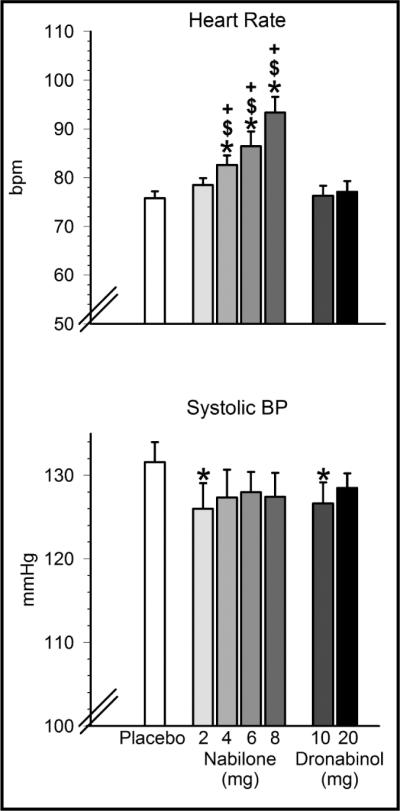
Effects of nabilone (2, 4, 6, 8 mg) and dronabinol (10, 20 mg) on heart rate (top) and systolic blood pressure (bottom) in regular marijuana smokers. Data are means of peak scores from seven assessments taken after capsule administration (± SEM). Asterisks denote a significant difference from placebo (p < 0.05) in the indicated condition. $ denotes a significant difference from dronabinol (10 mg). + denotes a significant difference from dronabinol (20 mg).
DISCUSSION
Nabilone, at acute doses higher than administered in previous studies (6 and 8 mg compared to 5 mg, the highest dose previously administered to marijuana smokers; Lile et al. 2010) and up to four times higher than doses given clinically for nausea, was well tolerated in this marijuana-using sample, as indicated by dose-related increases in self-reported positive drug effects and few negative effects. Nabilone caused orderly increases in heart rate that did not require medical intervention in this healthy sample. Although the increases observed on the higher dose of nabilone (8 mg), which in some individuals exceeded 100 beats per minute (up to 119 bpm) could be medically significant in a clinical population, on nabilone (4 mg) no individual's heart rate exceeded 100 beats per minute at any time point, and nabilone (6 mg) produced only one reading over 100 bpm (106 bpm), suggesting limited cardiovascular risk at these doses. Effects on cognitive function were minor, with the main finding being modest decreases in psychomotor speed at the highest doses of nabilone (6, 8 mg).
The lowest nabilone dose (2 mg) had few effects overall, whereas the 4 mg dose most closely mirrored dronabinol's (10, 20 mg) effects; these findings are consistent with earlier findings showing that nabilone (3, 5 mg) substituted for dronabinol (25 mg) in a drug discrimination paradigm (Lile et al. 2010). In the current study, higher doses of nabilone (6, 8 mg) produced the most robust positive subjective effects, disruption in cognitive performance, and cardiovascular changes. Importantly, the positive drug effects produced by both nabilone and dronabinol were significant, but even these high doses did not compare to the peak ratings of `high' produced by three puffs of low-potency smoked marijuana (e.g. Haney 2002; Haney et al. 1999b; Hart et al. 2001). In addition, the effects of nabilone were markedly more sustained than the effects of smoked marijuana (Haney 2002; Haney et al. 1999b; Hart et al. 2001), supporting its possible utility as a treatment for marijuana dependence. This is consistent with earlier reports of prolonged effects of nabilone in healthy volunteers (Lemberger and Rowe 1975). In the present study, the effects of nabilone were still apparent five hours after capsule administration. Although the session length was insufficient to determine whether nabilone's effects were longer lasting than those of dronabinol, observation of the data (for example, see Figure 2), suggest that this might be the case. Future research should address this possibility.
The present results are broadly consistent with earlier studies investigating the effects of nabilone in humans, after accounting for methodological differences. A prior laboratory study indicated that a low dose of nabilone (2 mg), while producing modest increases in subjective reports of feeling `high', did not increase `elation' ratings in daily, weekly, or occasional marijuana smokers (Mendelson and Mello 1984), consistent with the present findings that the lower dose of nabilone did not produce positive mood effects. Whereas an earlier study reported that lower doses of nabilone (1–3 mg) produced marked cognitive impairments (Wesnes et al. 2010), we found only modest decreases in psychomotor and potentially information processing speed. The most parsimonious explanation for this difference is that our sample consisted of near-daily marijuana smokers who would be expected to demonstrate tolerance to the effects of cannabinoids, whereas the earlier study employed non-marijuana smoking volunteers. We have previously reported the cognitive effects of smoked and oral cannabinoids in regular marijuana smokers to be mild (Bedi et al. 2010; Hart et al. 2010; Hart et al. 2001). This earlier study (Wesnes et al. 2010) also reported common and sometimes severe adverse effects of nabilone such as high levels of anxiety and aggression, and loss of consciousness. In the present study, however, the doses employed did not produce substantial adverse effects, despite being almost three times higher than the highest doses employed by Wesnes et al. (2010), further supporting tolerance to these effects in our clinically-relevant sample.
Although the present study did not measure the reinforcing effects of nabilone or dronabinol, a recent review of evidence from Canada, where nabilone has been approved for some 30 years for chemotherapy-related nausea, failed to reveal substantial evidence of diversion and concluded that nabilone abuse appears to be rare (Ware and St Arnaud-Trempe 2010). Moreover, in a human laboratory study evaluating the reinforcing effects of a low dose of nabilone (2 mg), no operant responding was observed for nabilone by daily, weekly, or occasional marijuana smokers (Mendelson and Mello 1984). However, it is noteworthy that this prior study (Mendelson and Mello 1984) examined a dose of nabilone (2 mg) that in the present study did not produce positive mood effects. Thus, it is possible that higher doses of nabilone may be more readily self-administered. Our findings that the 6 and 8 mg doses of nabilone increased ratings of drug likeability and willingness to take the drug again would support direct investigation of their reinforcing effects in regular marijuana users.
There are numerous other avenues for future research. It remains to be seen whether nabilone decreases withdrawal symptoms in abstinent heavy marijuana users, as has been demonstrated to be the case for dronabinol (Budney et al. 2007b; Haney et al. 2004; Levin et al. 2011). In the present study there was no effect of nabilone on craving for marijuana (see Table 1) however participants had been abstinent for fewer than 24 hours. Marijuana withdrawal symptoms typically emerge after some 24 hours of abstinence, suggesting that nabilone's effects on withdrawal symptoms, including craving, might require a longer period of abstinence to become apparent. We have previously reported that dronabinol decreases marijuana craving during withdrawal (Haney et al. 2004), suggesting that nabilone may have a similar effect. A prior study (Lile et al. 2011) has shown that lower doses of nabilone (1, 3 mg) are well tolerated in combination with dronabinol (5, 15, 30 mg). Future studies should determine how nabilone influences the direct effects of smoked marijuana as well as how nabilone influences marijuana withdrawal and relapse. These data can then inform clinical testing of this approach in treatment-seeking marijuana users.
Although dronabinol has shown promise in reducing withdrawal symptoms in abstinent marijuana users (Budney et al. 2007b; Haney et al. 2004; Levin et al. 2011), to date it has not proven effective in reducing relapse rates (Haney et al. 2008; Levin et al. 2011). The absence of clear dose-dependence in dronabinol's effects in this and earlier studies (e.g. Haney et al. 1999a), which may relate to its variable metabolism and low bioavailability (Ben Amar 2006; McGilveray 2005), may have contributed to its failure to reduce relapse rates in studies thus far. Nabilone, which is better absorbed, has a longer time to peak effects, and has urinary metabolites that are distinct from those of marijuana, may thus be a superior medication with which to investigate the clinical utility of agonist treatment in marijuana dependence. The present data support nabilone as a promising candidate medication and demonstrate the importance of further investigation of nabilone as a potential treatment for marijuana dependence.
Acknowledgements
The authors would like to thank Michael Harakas for assistance in data collection. This research was supported by the National Institute on Drug Abuse (DA09236). This study complies with the laws of the country in which it was performed (USA). The authors have no conflict of interest to declare. (USA).
Footnotes
The authors have no conflict of interest to declare.
Authors' contribution: MH was responsible for the study concept and design. GB, ZC, and MH contributed to the acquisition of data. GB performed the data analysis. MH assisted with data analysis and interpretation of findings. GB drafted the manuscript. MH and ZC provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- APA . DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; MD: 1994. [Google Scholar]
- Bedi G, Foltin R, Gunderson E, Rabkin J, Hart C, Comer S, Vosburg S, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: A controlled laboratory study. Psychopharmacology. 2010;212:675–86. doi: 10.1007/s00213-010-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Brook DW. Developmental trajectories of marijuana use from adolescence to adulthood. Arch Pediatr Adolesc Med. 2011;165:55–60. doi: 10.1001/archpediatrics.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney A, Roffman R, Stephens R, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007a;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–16. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes J, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007b;86:22–9. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB, Davies M. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug Alcohol Depend. 1997;46:53–67. doi: 10.1016/s0376-8716(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fraser A, Meatherall R. Lack of interference by Nabilone in the EMIT® d.a.u cannabinoid assay, Abbott TDx® cannabinoid assay, and a sensitive TLC assay for delta-9-THC-carboxylic acid. J Anal Toxicol. 1989;13:240. doi: 10.1093/jat/13.4.240. [DOI] [PubMed] [Google Scholar]
- Grassi MC, Enea D, Ferketich AK, Lu B, Pasquariello S, Nencini P. Effectiveness of varenicline for smoking cessation: a 1-year follow-up study. J Subst Abuse Treat. 2011;41:64–70. doi: 10.1016/j.jsat.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Haney M. Effects of smoked marijuana in healthy and HIV + marijuana smokers. J Clin Psychopharmacol. 2002;42:34S–40S. doi: 10.1002/j.1552-4604.2002.tb06001.x. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: Differences between marijuana smokers and non-marijuana smokers. Neuropsychopharmacology. 2007;32:1391–403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: Benefits and pitfalls. Addict Biol. 2008;14:9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart C, Vosburg S, Comer S, Reed S, Foltin R. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197:157–68. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart C, Vosburg S, Nasser J, Bennett A, Zubaran C, Foltin R. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–70. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson E, Foltin R. Dronabinol and marijuana in HIV(+) marijuana smokers: Acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–8. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward A, Comer S, Foltin R, Fischman M. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999a;141:385–94. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward A, Comer S, Foltin R, Fischman M. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart C, Haney M, Vosburg S, Comer S, Foltin R. Reinforcing effects of oral Delta-9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–43. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Hart C, Ilan A, Gevins A, Gunderson E, Role K, Colley J, Foltin R. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96:333–41. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C, van Gorp W, Haney M, Foltin R, Fischman M. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–65. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–36. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. 4th edn. Prentice Hall; Upper Saddle River, NJ: 1991. [Google Scholar]
- Lemberger L, Rowe H. Clinical pharmacology of nabilone, a cannabinol derivative. Clin Pharmacol Ther. 1975;18:720–6. doi: 10.1002/cpt1975186720. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Rubin A, Wolen R, DeSante K, Rowe H, Forney R, Pence P. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev. 1982;9:17–23. doi: 10.1016/s0305-7372(82)80031-5. [DOI] [PubMed] [Google Scholar]
- Levin F, Mariani J, Brooks D, Pavlicova M, Cheng W, Nunes E. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–50. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile J, Kelly T, Hays L. Substitution profile of the cannabinoid agonist nabilone in human subjects discriminating delta-9-Tetrahydrocannabinol. Clin Neuropharmacol. 2010;33:235–42. doi: 10.1097/WNF.0b013e3181e77428. [DOI] [PubMed] [Google Scholar]
- Lile J, Kelly T, Hays L. Separate and combined effects of the cannabinoid agonists nabilone and delta9-THC in humans discriminating delta9-THC. Drug Alcohol Depend. 2011;116:86–92. doi: 10.1016/j.drugalcdep.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilveray I. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Mello N. Reinforcing effects of oral delta-9 tetrahydrocannabinol, smoked marijuana, and nabilone: Influence of previous marijuana use. Psychopharmacology. 1984;83:351–56. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- MTP Research Group Brief treatments for cannabis dependence: Findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–66. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32:503–17. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- SAMSHA . Results from the 2009 National Survey on Drug Use and Health. SAMSHA, Office of Applied Statistics; Rockville, MD: 2010. [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 2nd edn. CRC Press LLC; Boca Raton, FL: 2000. [Google Scholar]
- Stapleton J, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: Randomized placebo-controlled trial. Addiction. 2011;106:824–32. doi: 10.1111/j.1360-0443.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- Threlkeld M, Parran T, Adelman C, Grey S, Yu J. Tramadol versus buprenorpine for the management of acute heroin withdrawal: A retrospective matched cohort control study. Am J Addict. 2006;15:186–91. doi: 10.1080/10550490500528712. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . 2007 World Drug Report. UNODC; 2007. [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware M, St Arnaud-Trempe E. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010;105:494–503. doi: 10.1111/j.1360-0443.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Annas P, Edgar C, Deeprose C, Karlsten R, Philipp A, Kalliomaki J, Segerdahl M. Nabilone produces marked impairments to cognitive function and changes in subjective state in healthy volunteers. J Psychopharmacol. 2010;24:1659–69. doi: 10.1177/0269881109105900. [DOI] [PubMed] [Google Scholar]
- Zobel I, Kech S, van Calker D, Dykierek P, Berger M, Schneibel R, Schramm E. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiat Scand. 2011;123:276–82. doi: 10.1111/j.1600-0447.2010.01671.x. [DOI] [PubMed] [Google Scholar]