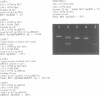
Abstract
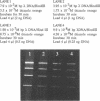
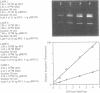
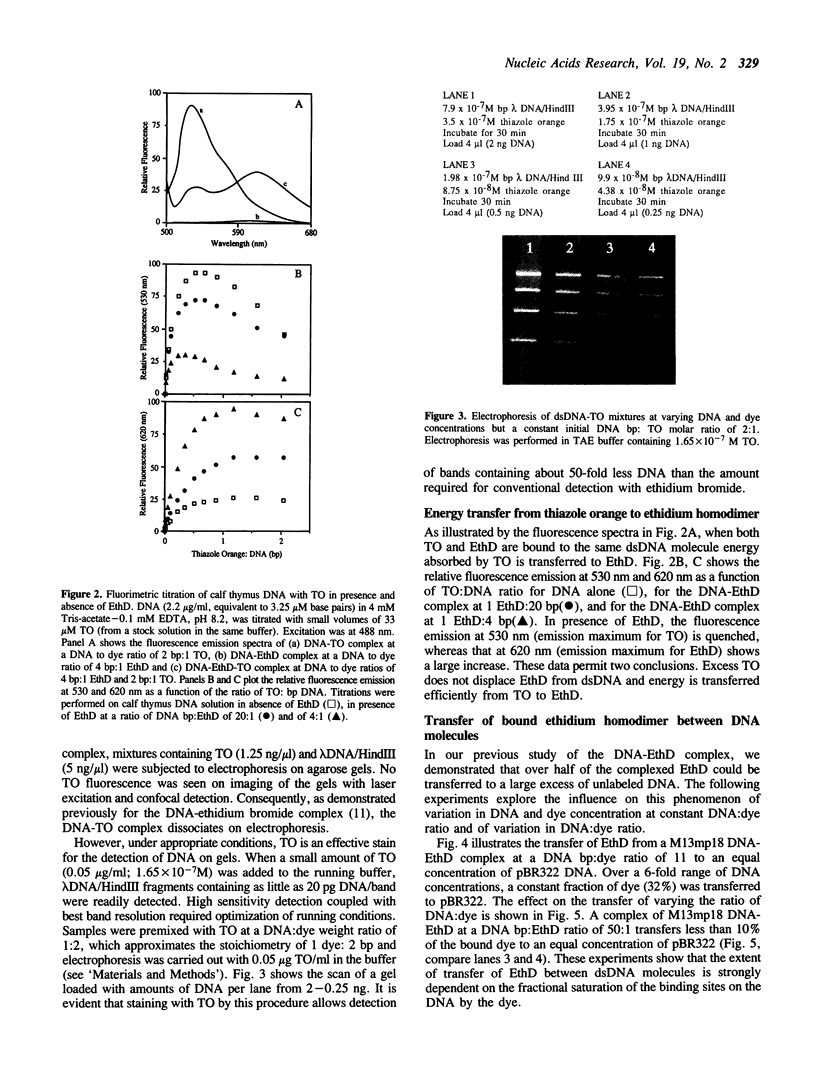
Ethidium homodimer (EthD; lambda Fmax 620 nm) at EthD:DNA ratios up to 1 dye:4-5 bp forms stable fluorescent complexes with double-stranded DNA (dsDNA) which can be detected with high sensitivity using a confocal fluorescence gel scanner (Glazer, A.N., Peck, K. & Mathies, R.A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3851-3855). However, on incubation with unlabeled DNA partial migration of EthD takes place from its complex with dsDNA to the unlabeled DNA. It is shown here that this migration is dependent on the fractional occupancy of intercalating sites in the original dsDNA-EthD complex and that there is no detectable transfer from dsDNA-EthD complexes formed at 50 bp: 1 dye. The monointercalator thiazole orange (TO; lambda Fmax 530 nm) forms readily dissociable complexes with dsDNA with a large fluorescence enhancement on binding (Lee, L.G., Chen, C. & Liu, L.A. (1986) Cytometry 7, 508-517). However, a large molar excess of TO does not displace EthD from its complex with dsDNA. When TO and EthD are bound to the same dsDNA molecule, excitation of TO leads to efficient energy transfer from TO to EthD. This observation shows the practicability of 'sensitizing' EthD fluorescence with a second intercalating dye having a very high absorption coefficient and efficient energy transfer characteristics. Electrophoresis on agarose gels, with TO in the buffer, of preformed linearized M13mp18 DNA-EthD complex together with unlabeled linearized pBR322 permits sensitive fluorescence detection in the same lane of pBR322 DNA-TO complex at 530 nm and of M13mp18 DNA-EthD complex at 620 nm. These observations lay the groundwork for the use of stable DNA-dye intercalation complexes carrying hundreds of chromophores in two-color applications such as the physical mapping of chromosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assa-Munt N., Denny W. A., Leupin W., Kearns D. R. 1H NMR study of the binding of Bis(acridines) to d(AT)5.d(AT)5. 1. Mode of binding. Biochemistry. 1985 Mar 12;24(6):1441–1449. doi: 10.1021/bi00327a024. [DOI] [PubMed] [Google Scholar]
- Assa-Munt N., Leupin W., Denny W. A., Kearns D. R. 1H NMR study of the binding of bis(acridines) to d(AT)5.d(AT)5. 2. Dynamic aspects. Biochemistry. 1985 Mar 12;24(6):1449–1460. doi: 10.1021/bi00327a025. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Delepierre M., Dinh T. H., Roques B. P. Bisintercalation of ditercalinium into a d(CpGpApTpCpG)2 minihelix: a 1H- and 31P-NMR study. Biopolymers. 1989 Dec;28(12):2115–2142. doi: 10.1002/bip.360281207. [DOI] [PubMed] [Google Scholar]
- Fan Y. S., Davis L. M., Shows T. B. Mapping small DNA sequences by fluorescence in situ hybridization directly on banded metaphase chromosomes. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6223–6227. doi: 10.1073/pnas.87.16.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. A., Manning G. S. Polyelectrolyte effects on site-binding equilibria with application to the intercalation of drugs into DNA. Biopolymers. 1984 Dec;23(12):2671–2714. doi: 10.1002/bip.360231202. [DOI] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Capelle N., Roques B. P., Le Pecq J. B. DNA Bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Oberlin R., Roques B. P., Le Pecq J. B. DNA bifunctional intercalators. I. Synthesis and conformational properties of an ethidium homodimer and of an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5071–5078. doi: 10.1021/bi00617a001. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Peck K., Mathies R. A. A stable double-stranded DNA-ethidium homodimer complex: application to picogram fluorescence detection of DNA in agarose gels. Proc Natl Acad Sci U S A. 1990 May;87(10):3851–3855. doi: 10.1073/pnas.87.10.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugâa P., Markovits J., Delbarre A., Le Pecq J. B., Roques B. P. DNA tris-intercalation: first acridine trimer with DNA affinity in the range of DNA regulatory proteins. Kinetic studies. Biochemistry. 1985 Sep 24;24(20):5567–5575. doi: 10.1021/bi00341a042. [DOI] [PubMed] [Google Scholar]
- Lee L. G., Chen C. H., Chiu L. A. Thiazole orange: a new dye for reticulocyte analysis. Cytometry. 1986 Nov;7(6):508–517. doi: 10.1002/cyto.990070603. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Markovits J., Roques B. P., Le Pecq J. B. Ethidium dimer: a new reagent for the fluorimetric determination of nucleic acids. Anal Biochem. 1979 Apr 15;94(2):259–264. doi: 10.1016/0003-2697(79)90357-9. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Zhen W. P., Henriksen U., Buchardt O. Sequence-influenced interactions of oligoacridines with DNA detected by retarded gel electrophoretic migrations. Biochemistry. 1988 Jan 12;27(1):67–73. doi: 10.1021/bi00401a012. [DOI] [PubMed] [Google Scholar]
- Pelaprat D., Delbarre A., Le Guen I., Roques B. P., Le Pecq J. B. DNA intercalating compounds as potential antitumor agents. 2. Preparation and properties of 7H-pyridocarbazole dimers. J Med Chem. 1980 Dec;23(12):1336–1343. doi: 10.1021/jm00186a010. [DOI] [PubMed] [Google Scholar]
- Rao S. N., Kollman P. A. Molecular mechanical simulations on double intercalation of 9-amino acridine into d(CGCGCGC) X d(GCGCGCG): analysis of the physical basis for the neighbor-exclusion principle. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5735–5739. doi: 10.1073/pnas.84.16.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin L. P. Polyfunctional DNA intercalating agents. Med Res Rev. 1986 Jul-Sep;6(3):275–340. doi: 10.1002/med.2610060303. [DOI] [PubMed] [Google Scholar]
- Winkle S. A., Rosenberg L. S., Krugh T. R. On the cooperative and noncooperative binding of ethidium to DNA. Nucleic Acids Res. 1982 Dec 20;10(24):8211–8223. doi: 10.1093/nar/10.24.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. G., Wakelin L. P., Fieldes A., Acheson R. M., Waring M. J. Effects of ring substituents and linker chains on the bifunctional intercalation of diacridines into deoxyribonucleic acid. Biochemistry. 1980 Dec 9;19(25):5825–5836. doi: 10.1021/bi00566a026. [DOI] [PubMed] [Google Scholar]