Abstract
N-methyl-D-aspartate (NMDA) receptors are ionotropic glutamate receptors that mediate excitatory neurotransmission. NMDA receptors are also important drug targets that are implicated in a number of pathophysiological conditions. To facilitate the transition from lead compounds in pre-clinical animal models to drug candidates for human use, it is important to establish whether NMDA receptor ligands have similar properties at rodent and human NMDA receptors. Here, we compare amino acid sequences for human and rat NMDA receptor subunits and discuss inter-species variation in the context of our current knowledge of the relationship between NMDA receptor structure and function. We summarize studies on the biophysical properties of human NMDA receptors and compare these properties to those of rat orthologs. Finally, we provide a comprehensive pharmacological characterization that allows side-by-side comparison of agonists, un-competitive antagonists, GluN2B-selective non-competitive antagonists, and GluN2C/D-selective modulators at recombinant human and rat NMDA receptors. The evaluation of biophysical properties and pharmacological probes acting at different sites on the receptor suggest that the binding sites and conformational changes leading to channel gating in response to agonist binding are highly conserved between human and rat NMDA receptors. In summary, the results of this study suggest that no major detectable differences exist in the pharmacological and functional properties of human and rat NMDA receptors.
Keywords: ionotropic glutamate receptor, NMDA, pharmacology, electrophysiology, structure-function
1. Introduction
NMDA receptors are involved in neuronal development, synaptic plasticity, learning, and memory in the mammalian central nervous system (Traynelis et al., 2010). NMDA receptors are implicated in a number of pathophysiological conditions, including neuropathic pain, epilepsy, stroke, neurodegenerative diseases (e.g. Parkinson’s, Huntington’s, and Alzheimer’s diseases), and psychiatric disorders (e.g. schizophrenia and major depression) (Hardingham and Bading, 2010; Kalia et al., 2008; Lau and Zukin, 2007; Skolnick et al., 2009; Traynelis et al., 2010). NMDA receptors have therefore been intensively studied for the past decades as potential drug targets for a number of neurological and psychiatric indications (Koller and Urwyler, 2010; Waxman and Lynch, 2005). Despite these efforts, only few compounds targeting these receptors have been converted from pre-clinically active compounds into drugs for human use. Lowaffinity un-competitive antagonists (i.e. use-dependent channel blockers) and allosteric modulators of NMDA receptors have demonstrated improved safety profiles compared to competitive antagonists, which have failed in the clinic due to their narrow therapeutic windows. The most recent NMDA receptor ligand to be approved for human use is the low-affinity channel blocker memantine (Alzheimer's disease) (Lipton, 2006). Negative allosteric modulators selective for the GluN2B NMDA receptor subunit could be beneficial in the treatment of traumatic brain injury, neuropathic pain, and treatment-resistant depression and are also associated with few adverse side effects (Traynelis et al., 2010). These compounds and novel ligands in clinical trials have demonstrated that NMDA receptor antagonist can be used for therapeutic gain, and suggest a huge potential of NMDA receptor ligands, both inhibitors and potentiators, in the treatment of several neurological and psychiatric conditions. In addition, increasingly precise anatomical localization of the different NMDA receptor subunits has reinforced the therapeutic rationale for the development of subunit-selective NMDA receptor ligands (e.g. see Hallett and Standaert, 2004).
Seven NMDA receptor subunits have been identified; the GluN1 subunit, four different GluN2 subunits (GluN2A–D), and two GluN3 subunits (GluN3A,B). NMDA receptors are assembled from two GluN1 subunits and two GluN2 subunits and are activated by simultaneous binding of glycine and glutamate to GluN1 and GluN2 subunits, respectively (reviewed in Traynelis et al., 2010). The four GluN2 subunits play different roles during neuronal development and in the adult central nervous system (e.g. see Monyer et al., 1994), and endow NMDA receptors with different biophysical and pharmacological properties (Monyer et al., 1994; Vicini et al., 1998; Yuan et al., 2009). GluN3 subunits are capable of assembling with GluN1-GluN2 to modify functional properties, but many details of expression, assembly, and physiological roles of GluN3 subunits in the central nervous system are still unclear (Low and Wee, 2010). In this study, we focus on the GluN1 and GluN2A–D subunits.
Several unique pharmacological and biophysical features, including the requirement for simultaneous binding of the co-agonists glycine and glutamate for activation, slow deactivation, voltage-dependent Mg2+ block, and high permeability to Ca2+, distinguish NMDA receptors from the other ionotropic glutamate receptors. The activities of a wide range of agonists, competitive antagonists, channel blockers and allosteric modulators are well-characterized across the different NMDA receptor subtypes (Dravid et al., 2007; Erreger et al., 2007; Hansen et al., 2008; Hansen et al., 2010a). Considering the therapeutic potential of NMDA receptor ligands, it is surprising that the vast majority of studies on pharmacological and functional properties of NMDA receptor subtypes have been conducted on recombinant rat NMDA receptors. In other words, there is an apparent lack of data from human NMDA receptors. Relatively few studies have described functional properties of heterologously expressed human NMDA receptors (Bednar et al., 2004; Claiborne et al., 2003; Curtis et al., 2003; Daggett et al., 1998; Feuerbach et al., 2010; Grimwood et al., 1996a; Grimwood et al., 1996b; Hess et al., 1996; Hess et al., 1998; Le Bourdelles et al., 1994; Priestley et al., 1996; Priestley et al., 1995; Steinmetz et al., 2002; Steinmetz et al., 2004; Usala et al., 2003; Varney et al., 1996; Wafford et al., 1995). Although the amino acid sequence is highly conserved between rat and human subunits, some variation exist that may nonetheless confer significant pharmacological and functional differences.
1.1 Comparison of amino acid sequences for rat and human NMDA receptor subunits
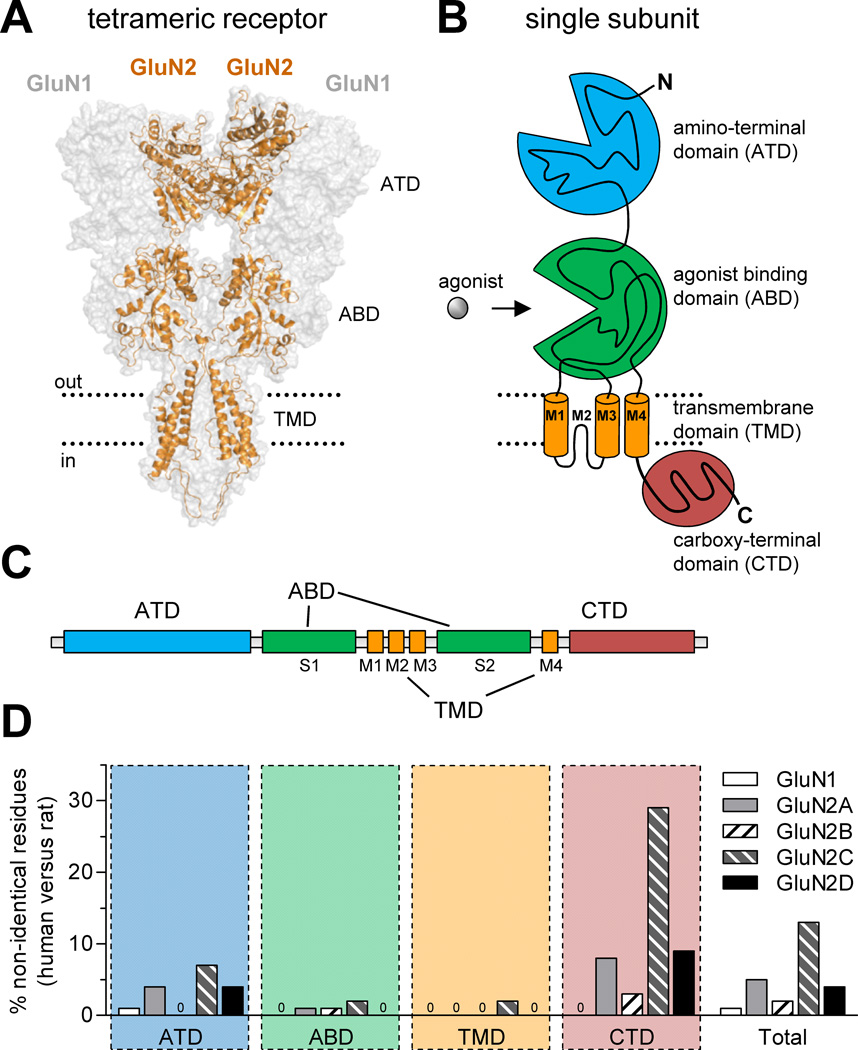
The overall sequence identities between rat and human orthologs of GluN1, GluN2A, GluN2B, GluN2C, and GluN2D are 99.3%, 95.3%, 98.5%, 87.1%, and 95.6%, respectively (Fig. 1 and Table 1). GluN1 shows high sequence identity between rat and human NMDA receptors with only 7 non-identical amino acids out of 938, whereas GluN2C is remarkably divergent with 162 non-identical amino acids out of 1236. The majority of variation between rat and human orthologs are located in the extracellular amino-terminal domain (ATD) and the intracellular carboxyl-terminal domain (CTD) (Fig. 1D and Table 1). Only few differences are located in the agonist binding and the transmembrane domains, suggesting conservation of key conformational changes that lead to channel gating in response to agonist binding. More detailed analyses of cDNAs and deduced amino acid sequences for human NMDA receptor subunits are described in the original cloning papers (Collins et al., 1993; Daggett et al., 1998; Foldes et al., 1994a; Foldes et al., 1993, 1994b; Hess et al., 1996; Hess et al., 1998; Karp et al., 1993; Le Bourdelles et al., 1994; Lin et al., 1996; Planells-Cases et al., 1993).
Figure 1.
Comparison of amino acid sequences for rat and human NMDA receptor subunits. (A) Model of the NMDA receptor based on the crystal structure of the membrane-spanning GluA2 AMPA receptor (Sobolevsky et al., 2009), showing the tetrameric arrangement of two GluN1 and two GluN2 subunits. The intracellular carboxyl-terminal domain (CTD) was not part of the crystal structure and is omitted from the model, which was generated as previously described (Acker et al., 2011). (B) NMDA receptor subunits consist of four semi-autonomous domains, namely the extracellular amino-terminal domain (ATD), the bi-lobed agonist binding domain (ABD), the transmembrane domain (composed of the transmembrane helices M1, M3, and M4, as well as the membrane re-entrant loop M2), and the intracellular carboxyl-terminal domain (CTD). (C) Linear representation of the polypeptide chain of NMDA receptor subunits, illustrating the relative positions of the four semi-autonomous domains in the amino acid sequence. The agonist binding domain (ABD) is formed by two amino acid segments termed S1 and S2. (D) The bar graph shows the percentage of residues that are not conserved between human and rat subunits for the four different domains and for the total protein. Insertion or deletions in human subunits compared to rat subunits are also categorized as non-identical positions. See Table 1 for more detail.
Table 1.
Comparison of amino acid sequences for human and rat NMDA receptor subunits.
| Signal peptide # of residues |
ATD # of residues |
ABD # of residues |
TMD # of residues |
CTD # of residues |
Total subunit # of residues |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| non- identical |
total | non- identical |
total | non- identical |
total | non- identical |
total | non- identical |
total | non- identical |
total | |
| GluN1 | 3 | 20 | 3 | 376 | 1 | 286 | 0 | 106 | 0 | 150 | 7 | 938 |
| GluN2A | 4 | 27 | 14 | 377 | 2 | 275 | 0 | 158 | 49 | 627 | 69 | 1464 |
| GluN2B | 2 | 28 | 1 | 376 | 2 | 276 | 0 | 158 | 18 | 646 | 23 | 1484 |
| GluN2C | 14 | 19 | 28 | 395 | 6 | 276 | 3 | 158 | 111 | 388 | 162 | 1236 |
| GluN2D | 0 | 25 | 15 | 402 | 0 | 277 | 0 | 158 | 44 | 474 | 59 | 1336 |
NMDA receptor subunits consist of the extracellular amino-terminal domain (ATD), the agonist binding domain (ABD), the transmembrane domain (TMD), and the intracellular carboxyl-terminal domain (CTD). Insertion or deletions in human subunits compared to rat subunits are also categorized as non-identical positions. ATD includes the ATD-S1 linker, and TMD is M1M2M3 including pre-M1 and intracellular loops. The ABD is S1 and S2 as well as S1-M1, M3-S2, and S2-M4 linkers. The amino acid sequences of all rat ionotropic glutamate receptor subunits were aligned using the ClustalW algorithm and the regions for each of the NMDA receptor subunits were determined based on GluA2 in this global alignment. In GluA2, the regions were defined as amino acids 1–397 (signal peptide and ATD), 398–414 (ATD-S1 linker), 415–527 (S1), 528–534 (S1-M1 linker), 535–647 (M1M2M3), 648–652 (M3-S2 linker), 653–794 (S2), 795–809 (S2-M4 linker), 810–833 (M4) and 834–884 (CTD). Signal peptides were predicted using the SignalP 3.0 server (Bendtsen et al., 2004). The GenBank IDs for amino acid sequences of human subunits used in the alignment are listed in Materials and Methods. NP_015566.1, NP_000824.1, NP_000825.2, NP_000826.1 (and NP_000826.2), and NP_000827.1 were used in the alignment for rat GluN1, GluN2A, GluN2B, GluN2C, and GluN2D, respectively.
The intracellular CTD varies greatly among different ionotropic glutamate receptor subunits. This domain may contain different phosphorylation sites and binding sites for intracellular proteins involved in regulation of membrane trafficking and receptor function (Traynelis et al., 2010). Deletion of the CTD of GluN1 and GluN2A does not abolish function, but does alter regulation (Ehlers et al., 1996; Kohr and Seeburg, 1996; Krupp et al., 1998; Vissel et al., 2001). The variation between CTDs of rat and human NMDA receptor subunits raises the possibility that differences might exist in membrane trafficking and phosphorylation, especially for GluN2C whose CTD is just 71% identical between rat and human orthologs (Fig. 1 and Table 1).
The ATD is a semi-autonomous domain that consists of the first ~400–450 residues starting at the N-terminus. Mutant NMDA receptor subunits, where the ATD has been completely removed, are capable of forming functional receptors (Gielen et al., 2009; Yuan et al., 2009). However, truncations of the ATD have been found to influence several key subunit-specific features of NMDA receptor function such as open probability, deactivation, desensitization (Gielen et al., 2009; Yuan et al., 2009). Thus, the ATD plays a regulatory role by influencing many of the differences in biophysical properties that exist among the NMDA receptor subtypes. Moreover, multiple NMDA receptor modulators bind the ATD (Hansen et al., 2010a), and some of the distinct pharmacological properties of different NMDA receptor subtypes are mediated by the GluN2 ATD (Gielen et al., 2009; Yuan et al., 2009). For example, the GluN2 ATD contributes to the difference in glutamate potency between GluN1/GluN2A and GluN1/GluN2D receptors (Yuan et al., 2009); glutamate potency at GluN1/GluN2A is ~10-fold lower than at GluN1/GluN2D. In addition, non-competitive antagonists, typified by the phenolethanolamines such as ifenprodil, bind to a site at the interface between the ATDs of GluN1 and GluN2B (Karakas et al., 2011). Since variations between ATDs of rat and human NMDA receptor subunits exist, there could be inter-species differences in biophysical and pharmacological properties.
1.2 Biophysical properties of rat and human NMDA receptors
NMDA receptors that contain different GluN2 subunits have different deactivation time course, single-channel conductance, and open probability (Table 2). For example, rat GluN1/GluN2A and GluN1/GluN2B receptors have higher mean single-channel conductance (47–48 pS) than rat GluN1/GluN2C and GluN1/GluN2D receptors (30–32 pS) (Table 2). The weighted time constants of deactivation (τdeactivation) following removal of glutamate also depends on the GluN2 subunit; τdeactivation for rat NMDA receptors are ~ 50 ms for GluN1/ GluN2A, ~300 ms for GluN1/GluN2B, ~400 ms for GluN1/ GluN2C, >2 seconds for GluN1/ GluN2D. In addition, rat NMDA receptors have different open probabilities depending on the GluN2 subunit; ~0.5 for GluN1/GluN2A, ~0.1 for GluN1/GluN2B, and <0.05 for GluN1/GluN2C and GluN1/GluN2D (Table 2).
Table 2.
Biophysical properties of human and rat NMDA receptor subtypes.
| NMDA receptor subtype | τdeactivation glutamate (ms) |
τdeactivation glycine (ms) |
Mean open time (ms) |
Mean single- channel conductance (pS) |
Open probability |
|
|---|---|---|---|---|---|---|
| GluN1/GluN2A | human | - | - | 2.2b | 40b | - |
| rat | 54k | 150h | 2.6d | 47d | 0.50l | |
| GluN1/GluN2B | human | - | - | 2.9b | 26b | - |
| rat | 287k | - | 2.8e | 48d | 0.12l | |
| GluN1/GluN2C | human | 440c | 510c | 0.7c | 21c | - |
| rat | 420g | 680h | 0.6d | 32d | <0.05g | |
| GluN1/GluN2D | human | 6300a | 1800a | 1.0a | 25a | - |
| rat | 3800j | 2500j | 1.1f | 30f | <0.05m | |
NMDA receptors are activated by saturating concentrations of glutamate and glycine. Mean open time and conductance for rat NMDA receptors are obtained using single-channel recordings, whereas these values for human NMDA receptors are obtained using noise analysis. All deactivation time constants are weighted time constants. Unless otherwise stated, data are from recombinant NMDA receptors expressed in HEK293 cells. - indicates data not available.
Hess et al., 1998; 2 mM extracellular Ca2+.
Varney et al., 1996; Ltk− cells, 2 mM extracellular Ca2+.
Daggett et al., 1998; 2 mM extracellular Ca2+.
Stern et al., 1992; Xenopus oocytes, 1mM extracellular Ca2+.
Banke and Traynelis, 2003; 0.7 mM extracellular Ca2+.
Wyllie et al., 1996; Xenopus oocytes, 0.85 mM extracellular Ca2+.
Dravid et al., 2008; 0.5 mM extracellular Ca2+.
Monyer et al., 1992; 1.8 mM extracellular Ca2+.
Hansen and Traynelis, 2011; 0.5 mM extracellular Ca2+.
Vicini et al., 1998; 2 mM extracellular Ca2+.
Erreger et al., 2005; 0.5 mM extracellular Ca2+.
Wyllie et al., 1998; Xenopus oocytes, 0.85 mM extracellular Ca2+.
Three studies have described the biophysical properties of human NMDA receptors (Daggett et al., 1998; Hess et al., 1998; Varney et al., 1996) (Table 2). Mean channel open times for human NMDA receptor subtypes are similar to those of the corresponding rat NMDA receptor subtypes. However, mean single-channel conductances for human NMDA receptor subtypes appear lower than those of the corresponding rat NMDA receptor subtypes. This difference in mean single-channel conductances likely reflects differences in experimental conditions. For example, values for mean single-channel conductance of rat NMDA receptors are directly measured using single-channel recordings, whereas these values for human NMDA receptors are estimated using noise analysis. In addition, increasing concentrations of extracellular Ca2+ reduces mean single-channel conductance (Dravid et al., 2008; Schorge et al., 2005; Wyllie et al., 1996), and single-channel conductances for human subtypes were obtained at higher extracellular Ca2+ (2 mM) than those for rat subtypes (0.85–1.0 mM). Deactivation time constants following removal of glutamate or glycine are similar for rat and human GluN1/GluN2C and GluN1/GluN2D receptors (Table 2). No data exist for deactivation of human GluN1/GluN2A and GluN1/GluN2B receptors. Furthermore, open probabilities of the different human NMDA receptor subtypes have not been determined. In summary, these results do not suggest marked inter-species differences in biophysical properties. However, the data for human NMDA receptors are incomplete and more work is required to rule out that differences exist between rat and human orthologs.
1.3 Pharmacology of human NMDA receptors
A number agonists and competitive antagonists have been characterized on human recombinant NMDA receptor subtypes using electrophysiological methods and fluorescence-based measurements of intracellular Ca2+ (Bednar et al., 2004; Daggett et al., 1998; Feuerbach et al., 2010; Hess et al., 1996; Hess et al., 1998; Le Bourdelles et al., 1994; Priestley et al., 1995; Varney et al., 1996; Wafford et al., 1995). The GluN2B-selective non-competitive antagonist ifenprodil has also been evaluated on human NMDA receptors with some variation in the obtained IC50-values, ranging from 110 nM to 2.2 µM at human GluN1/GluN2B (Bednar et al., 2004; Feuerbach et al., 2010; Hess et al., 1998; Varney et al., 1996). No studies have provided IC50 values at human NMDA receptors for un-competitive antagonist (i.e. voltage-dependent channel blockers) using electrophysiological methods, which is a prerequisite to control membrane potential. Finally, the pharmacological properties of rat and human NMDA receptors have not yet been compared in the same study. Here, we address the lack of comparative data for rat and human NMDA receptor subtypes by providing quantitative evaluation of the actions of agonists, un-competitive antagonists, GluN2B-selective non-competitive antagonists, and newly described GluN2C/D-selective modulators using two-electrode voltage-clamp recordings.
2. Materials and Methods
2.1 DNA constructs
Wild type rat cDNAs for GluN1-1a (GenBank accession numbers U11418 and U08261; hereafter GluN1), GluN2A (D13211), GluN2B (U11419), GluN2C (M91563), and GluN2D (L31611) were provided by Drs. S. Heinemann (Salk Institute), S. Nakanishi (Kyoto University), and P. Seeburg (University of Heidelberg). The full open reading frames of human NMDA receptor subunits were assembled from cDNA fragments of obtained from the I.M.A.G.E. Consortium (Carlsbad, CA) and Origene (Rockville, MD). High GC-content in the open reading frames of human GluN2C and GluN2D subunits was reduced in order to increase expression in Xenopus oocytes (Hess et al., 1998; Monyer et al., 1994). Briefly, the GC-content of cDNAs encoding the first 229 amino acids of human GluN2C and the first 118 amino acids of human GluN2D were reduced without changing the amino acid sequences by replacing this fragment with a custom-synthesized DNA fragment (Genscript USA Inc, Piscataway, NJ). Similarly, the GC-content was reduced for cDNAs of human GluN2C encoding amino acids 956–1236 and human GluN2D encoding amino acids 997–1336. In addition, both the length and GC-content of 5’ untranslated regions of GluN1, GluN2C, and GluN2D were reduced. The final cDNAs encode protein sequences corresponding to wild type human NMDA receptor subunits GluN1-1a (GenBank NP_015566), GluN2A (GenBank NP_000824), GluN2B (GenBank NP_000825), GluN2C (intermediate between GenBank NP_000826.1 [EPP insertion at amino acid position 1048 is present] and GenBank NP_000826.2 [arginine residue at position 1212 is present]), and GluN2D (GenBank NP_000827.1).
2.2 Ligands
L-glutamic acid, glycine, and S-(+)-ketamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). NMDA, memantine hydrochloride, ifenprodil ((1R*,2S*)-erythro-2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemitartrate), eliprodil (α-(4-chlorophenyl)- 4-[(4-fluorophenyl)methyl]-1-piperidineethanol), Ro 25–6981 ((αR,βS)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidinepropanol maleate), and clobenpropit (N-(4-chlorobenzyl)-S-[3-(4(5)-imidazolyl)propyl]isothiourea dihydrobromide) were purchased from Tocris Bioscience (Ellisville, MO). CIQ ((3-chlorophenyl) [3,4-dihydro-6,7-dimethoxy-1-[(4-methoxyphenoxy)methyl]-2(1H)-isoquinolinyl]methanone)) was obtained from Tocris Bioscience and synthesized as previously described (Mullasseril et al., 2010). QNZ46 ((E)-4-(6-methoxy-2-(3-nitrostyryl)-4-oxoquinazolin-3(4H)-yl)-benzoic acid) was synthesized as previously described (Mosley et al., 2010).
2.3 Two-electrode voltage-clamp recordings from Xenopus oocytes
For expression in Xenopus oocytes, DNA constructs were linearized by restriction enzymes to produce cRNAs using the mMessage mMachine kit (Ambion). Injection of cRNA and two-electrode voltage-clamp recordings from Xenopus laevis oocytes were performed as previously described (Traynelis et al., 1998). Injected oocytes were maintained at 15°C in Barth’s solution containing (in mM) 88 NaCl, 2.4 NaHCO3, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 5 Tris-HCl (pH 7.4 with NaOH). Recordings were performed 3–4 days post-injection at room temperature (23°C). The extracellular oocyte recording solution contained (in mM) 90 NaCl, 1 KCl, 10 HEPES, 0.5 BaCl2, 0.01 EDTA (pH 7.4 with NaOH). Voltage and current electrodes were filled with 0.3 and 3.0 M KCl, respectively, and current responses were recorded at a holding potential of −40 mV. Data acquisition and voltage control were accomplished with a two-electrode voltage-clamp amplifier (OC725, Warner Instruments, Hamden, CT).
2.4 Data Analysis
Multiple alignments of peptide sequences were performed using the ClustalW algorithm (AlignX, Vector NTI Advance 11, Invitrogen, Carlsbad, CA). Concentration–response data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Data for individual oocytes were fitted to the Hill equation using variable slope. Fitted EC50 or IC50 values and Hill coefficients (nH) from individual oocytes were used to calculate the mean and standard error of the mean (SEM). For graphical presentation, data points from individual oocytes were normalized to the maximum current response in the same recording and averaged. The averaged data points were then fitted to the Hill equation and plotted together with the resulting curve. Unpaired t-test (two-tailed) was used for statistical comparison of log EC50 and log IC50 values as indicated (P < 0.05 was considered significant). All data are presented as mean ± SEM.
3. Results
3.1 Characterization of NMDA receptor agonists
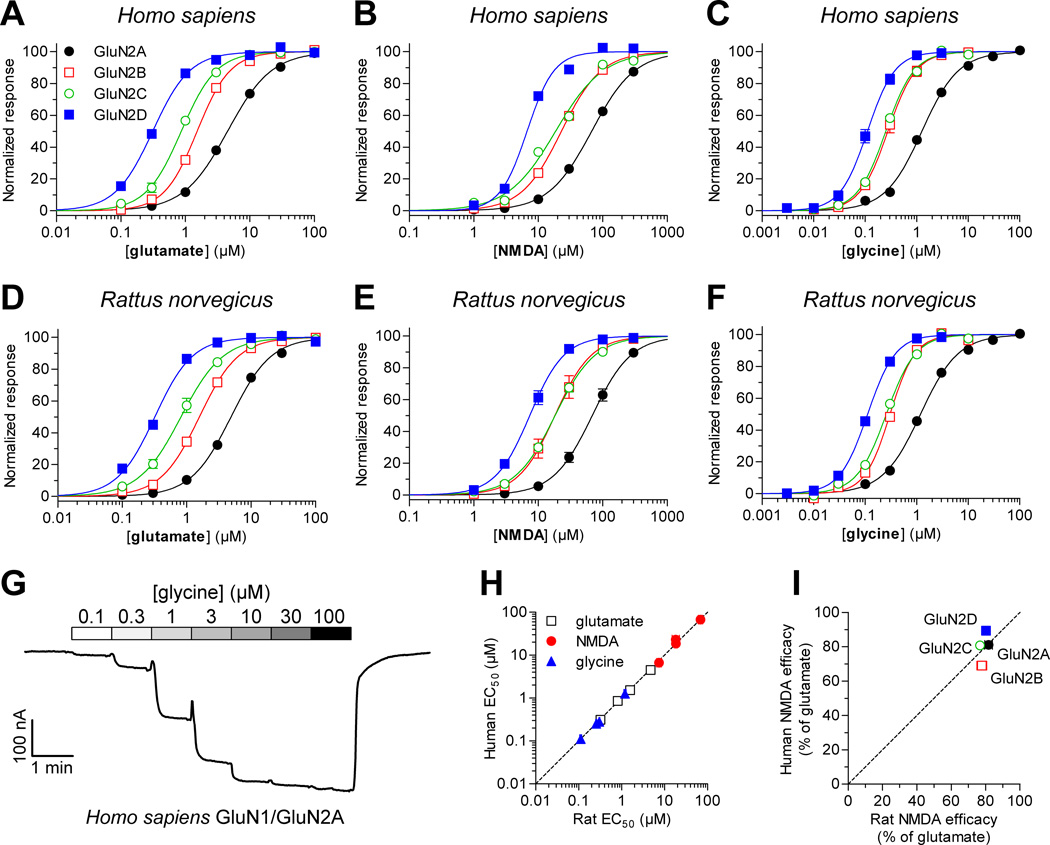
We generated concentration-response data for the two endogenous agonists glutamate and glycine at human and rat NMDA receptor subtypes expressed in Xenopus oocytes using two-electrode voltage-clamp electrophysiology. In addition, concentration-response data was generated for the partial GluN2 agonist NMDA. Data for agonists are summarized in Figure 2 and Table 3. EC50 values at human NMDA receptor subtypes were not significantly different than EC50 values at the corresponding rat NMDA receptor subtypes (P > 0.05, unpaired t-test). Furthermore, there was significant correlation between EC50 values at human and rat NMDA receptor subtypes for all three agonists (Fig. 2H; Pearson test, P < 0.05, r = 0.99 for all agonists). The rank order of potencies at the different subtypes was the same for all three agonists, namely GluN1/GluN2D > GluN1/GluN2C > GluN1/GluN2B > GluN1/GluN2A. This rank order of potencies is in agreement with previously published results from recombinant rat NMDA receptors (Chen et al., 2008; Erreger et al., 2007; Hansen et al., 2008). Maximal activation by the partial agonist NMDA was 69–89% compared to maximal activation by the full agonist glutamate and was similar for human and rat subtypes (Fig. 2I). The relative agonist efficacies of NMDA at human subtypes were not significantly correlated with relative efficacies at rat subtypes (Fig. 2I; Pearson test, P > 0.05, r = 0.46). However, this analysis is complicated by the narrow range of observed efficacies (69–89%). In summary, the results demonstrate that the activities of the GluN1 agonist glycine and the GluN2 agonists glutamate and NMDA are highly similar on human and rat NMDA receptors, suggesting indistinguishable agonist binding affinities and conservation of the conformational changes that lead to channel gating in response to agonist binding.
Figure 2.
Concentration-response data for agonists at human and rat NMDA receptors. Determination of EC50 values for glutamate, NMDA, and glycine at recombinant human (A–C) and rat (D–F) NMDA receptors expressed Xenopus oocytes was performed using two-electrode voltage-clamp electrophysiology. Responses are normalized to maximal currents to the ligand in the same recording. EC50 values are listed in Table 3. Glutamate and NMDA were applied in the continuous presence of 30 µM glycine, and glycine was applied in the continuous presence of 100 µM glutamate. (G) Representative two-electrode voltage-clamp recording of responses from human GluN1/GluNA receptors to increasing concentrations of glutamate in the continuous presence of 30 µM glycine. (H) EC50 values at human and rat NMDA receptor subtypes were significantly correlated for all three agonists (Pearson test, P < 0.05, r = 0.99 for all agonists). The dashed line represents the situation where EC50 values at human and rat receptors are equal. (I) Maximal activation by the partial agonist NMDA was 69–89% compared to maximal activation by the full agonist glutamate in the same recording and was similar for human and rat subtypes.
Table 3.
Agonist and positive modulator data for human and rat NMDA receptor subtypes.
| GluN1/GluN2A | GluN1/GluN2B | GluN1/GluN2C | GluN1/GluN2D | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agonists | EC50 (µM) |
nH | N | EC50 (µM) |
nH | N | EC50 (µM) |
nH | N | EC50 (µM) |
nH | N | |
| glutamate | human | 4.5 ± 0.3 | 1.3 | 5 | 1.5 ± 0.1 | 1.8 | 7 | 0.87 ± 0.05 | 1.6 | 6 | 0.31 ± 0.01 | 1.5 | 4 |
| rat | 4.8 ± 0.3 | 1.4 | 6 | 1.6 ± 0.1 | 1.5 | 7 | 0.83 ± 0.10 | 1.4 | 6 | 0.32 ± 0.02 | 1.5 | 3 | |
| NMDA | human | 68 ± 3 | 1.3 | 4 | 23 ± 2 | 1.4 | 4 | 19 ± 1 | 1.2 | 4 | 6.7 ± 0.2 | 2.1 | 4 |
| rat | 71 ± 9 | 1.5 | 7 | 21 ± 4 | 1.7 | 7 | 19 ± 2 | 1.4 | 10 | 7.9 ± 0.9 | 1.7 | 9 | |
| glycine | human | 1.3 ± 0.1 | 1.3 | 6 | 0.29 ± 0.04 | 1.7 | 5 | 0.25 ± 0.01 | 1.6 | 6 | 0.12 ± 0.01 | 1.8 | 7 |
| rat | 1.2 ± 0.1 | 1.2 | 8 | 0.30 ± 0.01 | 1.8 | 5 | 0.26 ± 0.01 | 1.5 | 7 | 0.11 ± 0.01 | 1.6 | 5 | |
| Positive allosteric modulator | |||||||||||||
| CIQ | human | N.E. | - | 4 | N.E. | - | 4 | 3.1 ± 0.2 | 1.6 | 8 | 3.5 ± 0.3 | 1.3 | 8 |
| rat | N.E. | - | 4 | N.E. | - | 4 | 6.2 ± 1.4* | 1.1 | 6 | 4.3 ± 0.4 | 1.3 | 5 | |
EC50 ± SEM for agonists and the positive allosteric modulator CIQ at recombinant NMDA receptors expressed in Xenopus oocytes using two-electrode voltage-clamp electrophysiology. nH is the Hill slope and N is the number of oocytes. Data for glutamate and NMDA were generated in the continuous presence of 30 µM glycine, and data for glycine was generated in the continuous presence of 100 µM glutamate. Data for CIQ was generated in the presence of 100 µM glutamate plus 30 µM glycine. N.E. indicates no effect at 30 µM CIQ.
indicates significantly different from EC50 at the corresponding human NMDA receptor subtype (P < 0.05, two-tailed, un-paired t test: logarithmic EC50 values were used for the test).
3.2 Characterization of un-competitive antagonists
Most un-competitive NMDA receptor antagonists are positively charged at physiological pH and function by blocking the cation-permeable pore when the channel opens. Thus, these channel blockers are use-dependent in that they bind to and block agonist-gated open channels more rapidly than closed channels. Furthermore, positively charged channel blockers are also voltage-dependent with increased inhibition at more negative membrane potentials. Uncompetitive NMDA receptor antagonists are neuroprotective in animal models of neurological disorders that involve excessive stimulation of the NMDA receptors, such as traumatic brain injury, epilepsy, and stroke (reviewed by Traynelis et al., 2010). However, clinical trials have not been successful due to dose-limiting side effects and a narrow temporal window for intervention. Interestingly, low affinity channel blockers that show fast blocking and unblocking kinetics are well-tolerated, and one such NMDA receptor antagonist have been approved for clinical use for the treatment of Alzheimer's disease (memantine) (Lipton, 2006). Another channel blocker ketamine is also approved for human use as an anesthetic agent (Annetta et al., 2005). Both ketamine and memantine are known to have antidepressant-like properties, which have motivated the development of NMDA receptor antagonists for treatment of depression (reviewed in Skolnick et al., 2009).
Here, we evaluated inhibition of recombinant human and rat NMDA receptor subtypes by ketamine and memantine (Table 4). Ketamine IC50 values were not significantly different between human and rat GluN1/GluN2A and GluN1/GluN2C (P > 0.05, unpaired t-test). However, ketamine IC50 values were 1.6- and 1.7-fold lower at rat GluN1/GluN2B and GluN1/GluN2D, respectively, compared to the corresponding human subtypes (P < 0.05, unpaired t-test). Ketamine IC50 values at human subtypes were not significantly correlated with IC50 values at rat subtypes (Pearson test, P > 0.05, r = 0.81). Memantine IC50 values were not significantly different between human and rat GluN1/GluN2A, GluN1/GluN2B, and GluN1/GluN2D (P > 0.05, unpaired t-test), but were 1.3-fold higher at rat GluN1/GluN2C compared to human GluN1/GluN2C (P < 0.05, unpaired t-test). Despite the inter-species difference for GluN1/GluN2C, there was significant correlation for memantine IC50 values at human and rat subtypes (Pearson test, P < 0.05, r = 0.99). The rank order of potencies at both human and rat subtypes was the same for both antagonists (GluN1/GluN2A > GluN1/GluN2B > GluN1/GluN2D > GluN1/GluN2C), and is in agreement with previously published results from recombinant rat NMDA receptors (Dravid et al., 2007). Although some inter-species differences were observed for ketamine and memantine IC50 values, the differences were less than 2-fold and were not observed at the same subtypes for the two antagonists. These results suggest that the clinically relevant un-competitive antagonists ketamine and memantine have highly similar activities on human and rat NMDA receptors.
Table 4.
Antagonist data for human and rat NMDA receptor subtypes.
| GluN1/GluN2A | GluN1/GluN2B | GluN1/GluN2C | GluN1/GluN2D | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Un-competitive antagonists |
IC50 (µM) |
nH | N | IC50 (µM) |
nH | N | IC50 (µM) |
nH | N | IC50 (µM) |
nH | N | |
| (+)ketamine | human | 3.2 ± 0.2 | 1.0 | 6 | 2.9 ± 0.3 | 1.0 | 4 | 0.79 ± 0.04 | 0.9 | 6 | 2.4 ± 0.1 | 0.9 | 4 |
| rat | 5.0 ± 0.9 | 1.0 | 6 | 1.8 ± 0.2* | 0.9 | 4 | 0.78 ± 0.13 | 1.0 | 6 | 1.4 ± 0.3* | 1.0 | 4 | |
| memantine | human | 4.1 ± 0.7 | 1.1 | 6 | 0.98 ± 0.06 | 1.0 | 5 | 0.43 ± 0.02 | 1.0 | 6 | 0.56 ± 0.04 | 0.9 | 6 |
| rat | 5.2 ± 0.7 | 1.1 | 4 | 0.95 ± 0.02 | 1.4 | 4 | 0.56 ± 0.05* | 0.9 | 5 | 0.56 ± 0.03 | 1.0 | 4 | |
| Subunit-selective antagonists | |||||||||||||
| ifenprodil | human | >100 | - | 4 | 0.13 ± 0.01 | 1.1 | 6 | N.E. | - | 4 | N.E. | - | 5 |
| rat | 52 ± 11 | 1.2 | 4 | 0.11 ± 0.01 | 1.2 | 5 | >100 | - | 4 | >100 | - | 5 | |
| eliprodil | human | N.E. | - | 5 | 0.93 ± 0.14 | 1.3 | 4 | N.E. | - | 4 | N.E. | - | 4 |
| rat | N.E. | - | 5 | 0.78 ± 0.09 | 1.2 | 6 | N.E. | - | 4 | N.E. | - | 6 | |
| Ro 25–6981 | human | N.E. | - | 4 | 0.049 ± 0.008 | 1.3 | 8 | N.E. | - | 5 | N.E. | - | 4 |
| rat | N.E. | - | 4 | 0.042 ± 0.006 | 1.3 | 4 | N.E. | - | 4 | N.E. | - | 4 | |
| clobenpropit | human | 8.1 ± 0.6 | 1.0 | 4 | 0.56 ± 0.05 | 1.0 | 5 | 9.8 ± 1.5 | 0.9 | 6 | 11 ± 1 | 1.0 | 8 |
| rat | 15 ± 1 | 1.6* | 4 | 0.56 ± 0.06 | 1.3 | 4 | 6.4 ± 0.6 | 0.8 | 4 | 9.7 ± 1.2 | 0.9 | 6 | |
| QNZ46 | human | >100 | - | 4 | >100 | - | 4 | 5.2 ± 0.2 | 1.0 | 7 | 3.6 ± 0.3 | 1.0 | 6 |
| rat | N.E. | - | 4 | >100 | - | 5 | 6.3 ± 0.3* | 1.2 | 6 | 3.8 ± 0.2 | 1.0 | 4 | |
IC50 ± SEM for antagonists at recombinant NMDA receptors expressed in Xenopus oocytes using two-electrode voltage-clamp electrophysiology. The membrane potential was clamped at −40 mV. nH is the Hill slope and N is the number of oocytes. Data for antagonists were generated in the presence of 100 µM glutamate plus 30 µM glycine. N.E. indicates less than 20% inhibition at 100 µM of the antagonist.
indicates significantly different from IC50 at the corresponding human NMDA receptor subtype (P < 0.05, two-tailed, un-paired t test: logarithmic IC50 values were used for the test).
3.3 Characterization of GluN2B-selective non-competitive antagonists
The phenylethanolamine ifenprodil was the first subunit-selective antagonist identified at any NMDA receptor subunit (Williams, 1993), and this compound and analogs have been invaluable pharmacological tools for the dissection of the physiological role of the GluN2B subunit in central nervous system (see (Hansen et al., 2010a). In general, GluN2B-selective antagonists are negative allosteric modulators (i.e. non-competitive antagonists) that bind the interface between GluN1 and GluN2B ATDs (Karakas et al., 2011). These antagonists have shown promising results in clinical trials as therapeutic agents in the treatment of traumatic brain injury, neuropathic pain, and treatment-resistant depression (Preskorn et al., 2008; Skolnick et al., 2009; Traynelis et al., 2010; Yurkewicz et al., 2005).
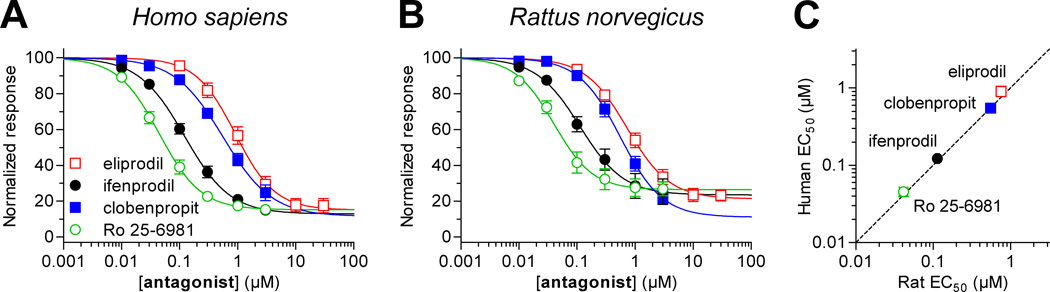
We evaluated inhibition of recombinant human and rat NMDA receptor subtypes by three GluN2B-selective antagonists, namely ifenprodil, eliprodil, and Ro 25–6981 (Fig. 3 and Table 4). In addition, the histamine H3 receptor antagonist clobenpropit was evaluated, since this compound is also a GluN2B-selective NMDA receptor antagonist with a dual channel-blocking (i.e. un-competitive) and non-competitive mechanism of action (Hansen et al., 2010b). IC50 values for all four antagonists were not significantly different between human and rat GluN1/GluN2B (P > 0.05, unpaired t-test). There was significant correlation between IC50 values at human and rat GluN1/GluN2B (Pearson test, P < 0.05, r = 0.99). Clobenpropit IC50 at rat GluN1/GluN2A was 1.9-fold higher than the IC50 at human GluN1/GluN2A (P < 0.05, unpaired t-test). The rank order of potencies at rat GluN1/GluN2B was Ro 25–6981 < ifenprodil < clobenpropit < eliprodil, which is in agreement with previously published results from recombinant rat NMDA receptors (Hansen et al., 2010a). The comparison of inhibition by GluN2B-selective antagonists at human and rat NMDA receptors did not identify any marked inter-species differences.
Figure 3.
Concentration-response data for GluN2B-selective non-competitive antagonists at human and rat NMDA receptors. Determination of IC50 values ifenprodil, eliprodil, clobenpropit, and Ro 25–6981 at recombinant human (A) and rat (B) GluN1/GluN2B receptors expressed Xenopus oocytes was performed using two-electrode voltage-clamp electrophysiology. IC50 values are listed in Table 4. Antagonists were applied in the presence of 100 µM glutamate plus 30 µM glycine. (C) IC50 values for GluN2B-selective antagonists at human and rat NMDA receptor GluN1/GluN2B receptors were significantly correlated (Pearson test, P < 0.05, r = 0.99).
3.4 Characterization of GluN2C/D-selective modulators
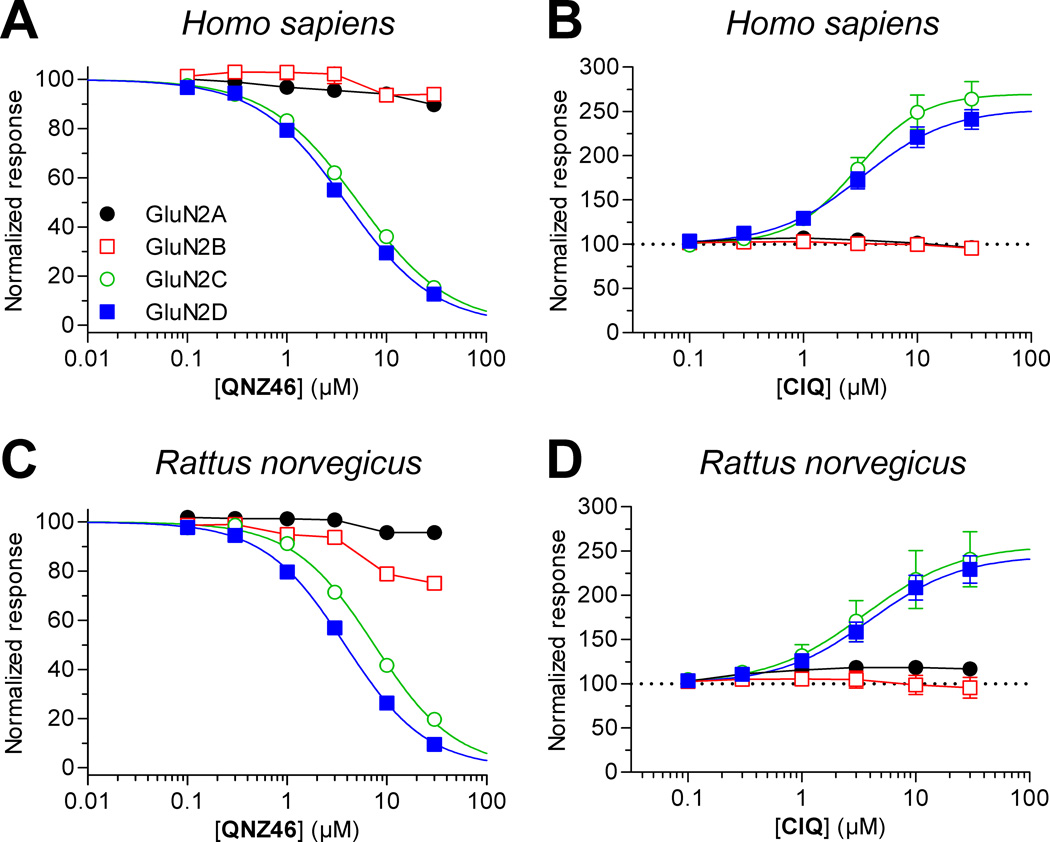
Several recent reports have described novel subunit-selective ligands for GluN2C- and GluN2D-containing receptors (Acker et al., 2011; Costa et al., 2010; Hansen and Traynelis, 2011; Mosley et al., 2010; Mullasseril et al., 2010). We evaluated inhibition of recombinant human and rat NMDA receptors by the GluN2C/D-selective non-competitive antagonist QNZ46 (Fig. 4 and Table 4). QNZ46 IC50 values at human and rat GluN1/GluN2D were not significantly different (P > 0.05, unpaired t-test), whereas QNZ46 IC50 at rat GluN1/GluN2C was marginally higher (1.2-fold) than at human GluN1/GluN2C (P < 0.05, unpaired t-test). We also evaluated potentiation of recombinant human and rat NMDA receptors by the GluN2C/D-selective positive allosteric modulator CIQ (Fig. 4 and Table 3). Similar to QNZ46, there were no significant difference between CIQ EC50 values at human and rat GluN1/GluN2D (P > 0.05, unpaired t-test), but CIQ EC50 at rat GluN1/GluN2C was 2.0-fold higher than at human GluN1/GluN2C (P < 0.05, unpaired t-test). Compared to NMDA receptor currents in the absence of CIQ, the maximal potentiation by CIQ was 270 ± 20 (N = 8) and 260 ± 30 (N = 6) at human and rat GluN1/GluN2C, respectively, and 250 ± 10 (N = 8) and 240 ± 20 (N = 5) at human and rat GluN1/GluN2D, respectively. Although the activities of QNZ46 and CIQ were highly similar on human and rat NMDA receptors, both modulators appeared slightly less potent at rat GluN1/GluN2C compared to the human ortholog.
Figure 4.
Concentration-response data for novel GluN2C/D-selective ligands at human and rat NMDA receptors. Determination of potencies for QNZ46 and CIQ at recombinant human (A,B) and rat (C,D) NMDA receptors expressed Xenopus oocytes was performed using two-electrode voltage-clamp electrophysiology. EC50 values for CIQ and IC50 values for QNZ46 are listed in Tables 3 and 4, respectively. Data points for GluN1/GluN2A and GluN1/GluN2B receptors could not be fitted to the Hill equation and are connected by straight lines. Ligands were applied in the presence of 100 µM glutamate plus 30 µM glycine.
4. Discussion
Here, we systematically compared the amino acid sequences for human and rat NMDA receptor subunits and show that inter-species variation is almost exclusively located in the extracellular ATD and the intracellular CTD with very little or no variation in agonist binding and transmembrane domains. Furthermore, we summarize previously published studies that describe biophysical properties of human NMDA receptors. The synthesis of our efforts on these two fronts suggests that inter-species differences in pharmacological and functional properties could exist for NMDA receptors and that more work is needed to substantiate this idea. Using two-electrode voltage-clamp electrophysiology, we compare pharmacological properties of recombinant rat and human NMDA receptors. However, we were not able to identify more than 2-fold differences in ligand potencies between human and rat NMDA receptor subtypes.
In addition to diheteromeric NMDA receptors composed of GluN1 and one type of GluN2 (e.g. GluN1/GluN2B), NMDA receptors can also assemble into triheteromeric receptors that contain more than one type of GluN2 subunit (e.g. GluN1/GluN2B/GluN2D) (e.g. (Jones and Gibb, 2005). Despite their potential importance in native neurons, little is known about how combinations of subunits with dissimilar properties impact pharmacology and of triheteromeric receptors. The variation between human and rat subunits located in the extracellular ATD, which is thought to mediate receptor assembly and regulate function (Hansen et al., 2010a), may result in differences in assembly of triheteromeric receptors in the human and rodent brain. Moreover, triheteromeric NMDA receptors may also have different functional properties in human versus rat neurons, a possibility that was not addressed in this study.
To improve the likelihood that NMDA receptor ligands with promising results in preclinical animal models can translate into drugs for human use, it is important to establish early in drug development whether these ligands have different pharmacological properties at rodent and human NMDA receptors. Despite this obvious caveat to the success of NMDA receptor ligands as drug candidates, no studies have directly compared human and rat NMDA receptor pharmacology. We addressed this problem and our results suggest that agonist binding affinities and conformational changes that lead to channel gating in response to agonist binding are highly conserved between human and rat NMDA receptors. Furthermore, the actions of NMDA receptor ligands that modulate receptor function through a diverse range of mechanisms and with marked differences in subunit-selectivity also appear to be highly conserved between human and rat receptors. These results suggest that no marked differences exist in the pharmacological and functional properties of human and rat NMDA receptors.
Highlights.
Variations between human and rat subunits are mainly located in the ATD and CTD.
Human and rat NMDA receptors have similar biophysical properties.
Agonist binding and channel gating appear conserved between human and rat receptors.
Non-competitive antagonists similarly inhibit human and rat NMDA receptors.
The molecular pharmacology of NMDA receptors is conserved between human and rat.
Acknowledgements
This work was supported by the National Institutes of Health-National Institute of Neurological Disorders and Stroke (SFT; NS036654, NS065371), the Lundbeck Foundation (KBH), the Villum Kann Rasmussen Foundation (KBH), and the GluTarget Programme of Excellence (HBO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC, Traynelis SF. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol. Pharmacol. 2011;80:782–795. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr. Drug Targets. 2005;6:789–794. doi: 10.2174/138945005774574533. [DOI] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat. Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- Bednar B, Cunningham ME, Kiss L, Cheng G, McCauley JA, Liverton NJ, Koblan KS. Kinetic characterization of novel NR2B antagonists using fluorescence detection of calcium flux. J. Neurosci. Methods. 2004;137:247–255. doi: 10.1016/j.jneumeth.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O'Toole KK, Le P, Lee CJ, Snyder JP, Traynelis SF, Wyllie DJ. Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J. Physiol. 2008;586:227–245. doi: 10.1113/jphysiol.2007.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne CF, McCauley JA, Libby BE, Curtis NR, Diggle HJ, Kulagowski JJ, Michelson SR, Anderson KD, Claremon DA, Freidinger RM, Bednar RA, Mosser SD, Gaul SL, Connolly TM, Condra CL, Bednar B, Stump GL, Lynch JJ, Macaulay A, Wafford KA, Koblan KS, Liverton NJ. Orally efficacious NR2B-selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:697–700. doi: 10.1016/s0960-894x(02)01061-2. [DOI] [PubMed] [Google Scholar]
- Collins C, Duff C, Duncan AM, Planells-Cases R, Sun W, Norremolle A, Michaelis E, Montal M, Worton R, Hayden MR. Mapping of the human NMDA receptor subunit (NMDAR1) and the proposed NMDA receptor glutamate-binding subunit (NMDARA1) to chromosomes 9q34.3 and chromosome 8, respectively. Genomics. 1993;17:237–239. doi: 10.1006/geno.1993.1311. [DOI] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. A novel family of negative and positive allosteric modulators of NMDA receptors. J. Pharmacol. Exp. Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis NR, Diggle HJ, Kulagowski JJ, London C, Grimwood S, Hutson PH, Murray F, Richards P, Macaulay A, Wafford KA. Novel N1-(benzyl)cinnamamidine derived NR2B subtype-selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:693–696. doi: 10.1016/s0960-894x(02)01060-0. [DOI] [PubMed] [Google Scholar]
- Daggett LP, Johnson EC, Varney MA, Lin FF, Hess SD, Deal CR, Jachec C, Lu CC, Kerner JA, Landwehrmeyer GB, Standaert DG, Young AB, Harpold MM, Velicelebi G. The human N-methyl-D-aspartate receptor 2C subunit: genomic analysis, distribution in human brain, and functional expression. J. Neurochem. 1998;71:1953–1968. doi: 10.1046/j.1471-4159.1998.71051953.x. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J. Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J. Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-Specific Agonist Activity at NR2A-, NR2B-, NR2C-, and NR2D-Containing N-Methyl-D-aspartate Glutamate Receptors. Mol. Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Loetscher E, Neurdin S, Koller M. Comparative pharmacology of the human NMDA-receptor subtypes R1-2A, R1-2B, R1-2C and R1-2D using an inducible expression system. Eur. J Pharmacol. 2010;637:46–54. doi: 10.1016/j.ejphar.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Foldes RL, Adams SL, Fantaske RP, Kamboj RK. Human N-methyl-D-aspartate receptor modulatory subunit hNR2A: cloning and sequencing of the cDNA and primary structure of the protein. Biochim. Biophys. Acta. 1994a;1223:155–159. doi: 10.1016/0167-4889(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Foldes RL, Rampersad V, Kamboj RK. Cloning and sequence analysis of cDNAs encoding human hippocampus N-methyl-D-aspartate receptor subunits: evidence for alternative RNA splicing. Gene. 1993;131:293–298. doi: 10.1016/0378-1119(93)90309-q. [DOI] [PubMed] [Google Scholar]
- Foldes RL, Rampersad V, Kamboj RK. Cloning and sequence analysis of additional splice variants encoding human N-methyl-D-aspartate receptor (hNR1) subunits. Gene. 1994b;147:303–304. doi: 10.1016/0378-1119(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Gielen M, Siegler RB, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S, Gilbert E, Ragan CI, Hutson PH. Modulation of 45Ca2+ influx into cells stably expressing recombinant human NMDA receptors by ligands acting at distinct recognition sites. J. Neurochem. 1996a;66:2589–2595. doi: 10.1046/j.1471-4159.1996.66062589.x. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Le BB, Atack JR, Barton C, Cockett W, Cook SM, Gilbert E, Hutson PH, McKernan RM, Myers J, Ragan CI, Wingrove PB, Whiting PJ. Generation and characterisation of stable cell lines expressing recombinant human N-methyl-D-aspartate receptor subtypes. J. Neurochem. 1996b;66:2239–2247. doi: 10.1046/j.1471-4159.1996.66062239.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol. Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Bräuner-Osborne H, Egebjerg J. Pharmacological characterization of ligands at recombinant NMDA receptor subtypes by electrophysiological recordings and intracellular calcium measurements. Comb. Chem. High Throughput Screen. 2008;11:304–315. doi: 10.2174/138620708784246040. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Furukawa H, Traynelis SF. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol. Pharmacol. 2010a;78:535–549. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Mullasseril P, Dawit S, Kurtkaya NL, Yuan H, Vance KM, Orr AG, Kvist T, Ogden KK, Le P, Vellano KM, Lewis I, Kurtkaya S, Du Y, Qui M, Murphy TJ, Snyder JP, Bräuner-Osborne H, Traynelis SF. Implementation of a Fluorescence-Based Screening Assay Identifies Histamine H3 Receptor Antagonists Clobenpropit and Iodophenpropit as Subunit-Selective N-Methyl-D-Aspartate Receptor Antagonists. J. Pharmacol. Exp. Ther. 2010b;333:650–662. doi: 10.1124/jpet.110.166256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Traynelis SF. Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J. Neurosci. 2011;31:3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess SD, Daggett LP, Crona J, Deal C, Lu CC, Urrutia A, Chavez-Noriega L, Ellis SB, Johnson EC, Velicelebi G. Cloning and functional characterization of human heteromeric N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 1996;278:808–816. [PubMed] [Google Scholar]
- Hess SD, Daggett LP, Deal C, Lu CC, Johnson EC, Velicelebi G. Functional characterization of human N-methyl-D-aspartate subtype 1A/2D receptors. J. Neurochem. 1998;70:1269–1279. doi: 10.1046/j.1471-4159.1998.70031269.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Gibb AJ. Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J. Physiol. 2005;569:209–221. doi: 10.1113/jphysiol.2005.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp SJ, Masu M, Eki T, Ozawa K, Nakanishi S. Molecular cloning and chromosomal localization of the key subunit of the human N-methyl-D-aspartate receptor. J. Biol. Chem. 1993;268:3728–3733. [PubMed] [Google Scholar]
- Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J. Physiol. 1996;492(Pt 2):445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M, Urwyler S. Novel N-methyl-D-aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin. Ther. Pat. 2010;20:1683–1702. doi: 10.1517/13543776.2010.533656. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron. 1998;20:317–327. doi: 10.1016/s0896-6273(00)80459-6. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Le Bourdelles B, Wafford KA, Kemp JA, Marshall G, Bain C, Wilcox AS, Sikela JM, Whiting PJ. Cloning, functional coexpression, and pharmacological characterisation of human cDNAs encoding NMDA receptor NR1 and NR2A subunits. J. Neurochem. 1994;62:2091–2098. doi: 10.1046/j.1471-4159.1994.62062091.x. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Bovetto S, Carver JM, Giordano T. Cloning of the cDNA for the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery. Brain Res. Mol. Brain Res. 1996;43:57–64. doi: 10.1016/s0169-328x(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Low CM, Wee KS. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Mol. Pharmacol. 2010;78:1–11. doi: 10.1124/mol.110.064006. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Bräuner-Osborne H, Liotta DC, Traynelis SF. Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J. Med. Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC, Traynelis SF. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat. Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Sun W, Ferrer-Montiel AV, Montal M. Molecular cloning, functional expression, and pharmacological characterization of an N-methyl-D-aspartate receptor subunit from human brain. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5057–5061. doi: 10.1073/pnas.90.11.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Macaulay AJ, Hill RG, Kemp JA. Electrophysiological characterisation of the antagonist properties of two novel NMDA receptor glycine site antagonists, L-695,902 and L-701,324. Neuropharmacology. 1996;35:1573–1581. doi: 10.1016/s0028-3908(96)00141-4. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le BB, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol. Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Schorge S, Elenes S, Colquhoun D. Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J. Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz RD, Fava E, Nicotera P, Steinhilber D. A simple cell line based in vitro test system for N-methyl-D-aspartate (NMDA) receptor ligands. J. Neurosci. Methods. 2002;113:99–110. doi: 10.1016/s0165-0270(01)00482-4. [DOI] [PubMed] [Google Scholar]
- Steinmetz RD, Firla B, Steinhilber D. Inhibition of the functional expression of N-methyl-D-aspartate receptors in a stably transformed cell line by cyclosporin A. Biochem. Pharmacol. 2004;68:563–571. doi: 10.1016/j.bcp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc. Biol. Sci. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J. Neurosci. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala M, Thompson SA, Whiting PJ, Wafford KA. Activity of chlormethiazole at human recombinant GABA(A) and NMDA receptors. Br.J. Pharmacol. 2003;140:1045–1050. doi: 10.1038/sj.bjp.0705540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney MA, Jachec C, Deal C, Hess SD, Daggett LP, Skvoretz R, Urcan M, Morrison JH, Moran T, Johnson EC, Velicelebi G. Stable expression and characterization of recombinant human heteromeric N-methyl-D-aspartate receptor subtypes NMDAR1A/2A and NMDAR1A/2B in mammalian cells. J. Pharmacol. Exp. Ther. 1996;279:367–378. [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JAH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Kathoria M, Bain CJ, Marshall G, Le BB, Kemp JA, Whiting PJ. Identification of amino acids in the N-methyl-D-aspartate receptor NR1 subunit that contribute to the glycine binding site. Mol. Pharmacol. 1995;47:374–380. [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil Discriminates Subtypes of the N-Methyl-D-Aspartate Receptor - Selectivity and Mechanisms at Recombinant Heteromeric Receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J. Physiol. 1998;510(Pt 1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc. Biol. Sci. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J. Neurosci. 2009;29:12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma. 2005;22:1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]