Abstract
Directional cellular movement is required for various organismal processes, including immune defense and cancer metastasis. Proper navigation of migrating cells involves responding to a complex set of extracellular cues, including diffusible chemical signals and physical structural information. In tissues, conflicting gradients and signals may require cells to not only respond to the environment but also modulate it for efficient adhesion formation and directional cell motility. Recently, we found that cells endocytose fibronectin (FN) and resecrete it from a late endosomal/lysosomal (LE/Lys) compartment to provide an autocrine extracellular matrix (ECM) substrate for cell motility. Branched actin assembly regulated by cortactin was required for trafficking of FN-containing vesicles from LE/Lys to the cell surface. These findings suggest a model in which migrating cells use lysosomal secretion as a versatile mechanism to modulate the ECM environment, promote adhesion assembly and enhance directional migration.
Keywords: Arp2/3 complex, branched actin, cell motility, cortactin, extracellular matrix, fibronectin, lamellipodium, late endosomal/lysosomal compartments, lysosomal secretion, migration
Introduction
Cellular movement requires dynamic reorganization of the internal cytoskeleton and is canonically described as occurring through four sequential processes: protrusion of leading edge lamellipodia, formation of new adhesions, cell body contraction, and tail detachment.1 These intrinsic processes are regulated in response to external cues, such as growth factors and extracellular matrix (ECM). Branched actin assembly is required for leading edge lamellipodial protrusion, the first step in the cycle. However, actin assembly is also critical for other cellular functions, including cell-cell adhesion and membrane trafficking, which could also affect cell motility.2
Many groups, including our own, have studied the role of the branched actin assembly protein cortactin in cell motility due to its presence in leading edge lamellipodia and its potential role in cancer metastasis.3 Detailed live cell analyses have indicated that cortactin is not required for lamellipodial protrusion but does affect the stability or persistence of lamellipodia after they are formed as well as the assembly of adhesions at the leading edge of migrating cells.4 Interestingly, similar defects in lamellipodial persistence as those exhibited by cortactin-knockdown (KD) cells are found in cells with primary defects in integrin levels or activity.5-8 Those data suggested to us that perhaps the lamellipodial persistence defects of cortactin-KD cells are a secondary consequence of adhesion assembly defects.
Cortactin Regulates Cell Motility by Promoting Secretion of ECM
Because cortactin is known to regulate membrane trafficking and exocytosis, we hypothesized that cortactin might promote cell motility, adhesion assembly and lamellipodial persistence as a direct consequence of enhancing ECM secretion. We began by determining whether cortactin regulates extrinsic or intrinsic mechanisms of cell motility by measuring the speed of cortactin-KD or overexpressing cells when plated on surfaces coated with various concentrations of ECM. Consistent with an extrinsic motility defect, cortactin-KD HT1080 human fibrosarcoma cells migrated poorly on uncoated surfaces but moved as rapidly as control cells on surfaces coated with high concentrations of exogenous ECM (10 μg/ml FN or 50 μg/ml Collagen I)-coated plates. Cortactin-KD cell defects in lamellipodial persistence and adhesion formation were also rescued by plating cells on 10 μg/ml FN. These findings suggested that cortactin might indeed regulate cell motility by promoting autocrine ECM secretion.
To directly test whether autocrine matrix from cortactin-expressing cells promotes cell motility, cell-free ECM was extracted from control, cortactin-KD and cortactin-KD cells rescued by reexpression of shRNA-insensitive wild-type cortactin (Rescue). In two different cell types, cell-free ECM extracted from control or Rescue cells supported higher motility of cortactin-KD cells than cell-free ECM extracted from cortactin-KD cells. Interestingly, control and Rescue cells migrated equivalently on control- and KD-produced ECM, suggesting that cortactin-expressing cells can dynamically secrete their own matrix during motility. Immunofluorescent staining of FN in autocrine produced cell-free ECM or deposited underneath cells (visualized by total internal reflection fluorescence (TIRF) microscopy) revealed that there was less FN deposition by cortactin-KD cells. Consistent with a primary defect in membrane trafficking, cortactin expression did not affect total cell levels of FN mRNA or protein; however, cortactin-KD cells exhibited a large accumulation of FN in the perinuclear region, suggesting an exit defect from a secretory compartment.
FN is Resecreted from a Late Endosomal/Lysosomal (LE/Lys) Compartment
To determine which secretory compartment is regulated by cortactin, we performed immunofluorescence colocalization studies with antibodies recognizing FN and various vesicular markers. Interestingly, FN colocalized well with the late endosomal marker Rab7 and the lysosomal marker LAMP1. In cortactin-KD cells there was an increase in both the colocalization of FN with Rab7 and LAMP1 and the size of the Rab7-positive compartment compared with control cells, suggesting a block in secretion from a late endosomal/lysosomal (LE/Lys) compartment.
Since FN is abundant in serum used in cell culture media9 and was highly colocalized with late endosomes in our system, we tested whether extracellular FN was endocytosed and resecreted by cells to promote cell motility. We removed FN from the serum used in cell culture media by affinity chromatography with gelatin-sepharose.10 After culturing cells in media containing the FN-depleted serum, we found that there was much less intracellular FN accumulation and the motility of all cortactin-manipulated cells (control, KD and rescue) was equivalent and dampened. Furthermore, live confocal and TIRF imaging using exogenously provided fluorescently-labeled FN revealed that the exogenous FN is internalized, contained in moving vesicles, and deposited at the basal surface of control cells. In cortactin-KD cells, FN was still internalized, but TIRF imaging revealed that there were fewer moving vesicles and less fluorescent FN deposited at the base of cells, consistent with the apparent trafficking block from LE/Lys compartments. KD of the lysosome-to-plasma membrane fusion regulator, synaptotagmin 7 (Syt7) also led to defective basal deposition of FN and motility, suggesting that secretory lysosomes may be an important vehicle for ECM deposition during cell motility.
Cortactin Regulates LE/Lys Secretion through Interactions with Branched Actin
To determine the underlying molecular interactions that mediate cortactin regulation of cell motility and FN secretion, cortactin-KD cells were rescued by reexpression of cortactin molecules with mutations in specific binding domains or phosphorylation sites. As is the case for many other cortactin-dependent phenotypes,3 binding to actin filaments and to the branched actin nucleating Arp2/3 complex was essential for cortactin to promote FN secretion and cell motility. By contrast, neither the tyrosine phosphorylation sites nor the SH3 domain was essential for these cortactin functions, suggesting that the most critical activity of cortactin in cell motility and FN secretion is to regulate branched actin networks. Given the specific block in FN trafficking at LE/Lys in cortactin-KD cells, these data suggest that dynamic branched actin assembly plays an important although unknown role in the generation of LE/Lys secretory vesicles.
Autocrine ECM Secretion Facilitates Effective Adhesion-Protrusion Cycles during Cell Motility
Adhesion assembly is a critical determinant of cell speed11 and directionality12 and requires binding of integrin receptors to ECM, activation of integrin-associated adhesion proteins and assembly of the actin cytoskeleton at adhesion sites. Our discovery that cells can regulate adhesion assembly and lamellipodial stability during cell motility by secreting ECM4,13 suggests an obvious mechanism by which cells might sharpen chemotactic responses and migrate more quickly. The impact of that secretion will of course depend on other environmental cues, but one could imagine a variety of in vivo situations in which the ability to modulate the surrounding environment would greatly facilitate physiologic migrations. Interestingly, although our work focused on FN, studies on Laminin-5 and collagen XVII in keratinocytes suggest that autocrine secretion of those ECM molecules may also facilitate migration and lamellipodial stability.5,14,15 Thus, we speculate that dynamic ECM secretion may be utilized by a variety of migratory cell types in diverse circumstances.
Lysosomal Secretion in Cell Motility
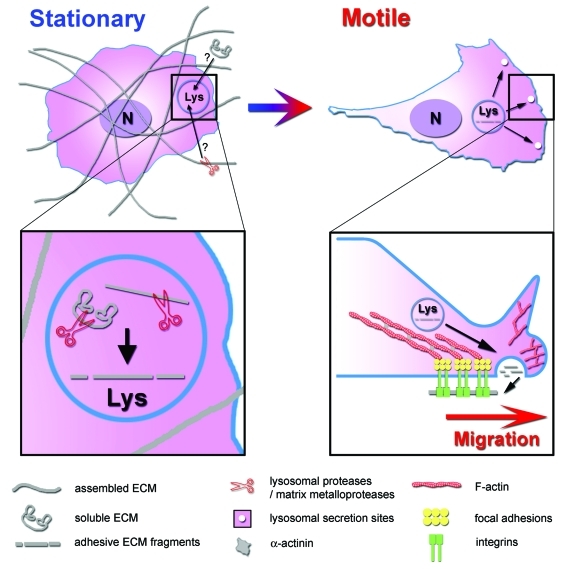
Our report indicates that lysosomal secretion is a route for FN deposition that promotes efficient cell motility. We speculate that trafficking of FN through LE/Lys may have advantages over classical Golgi secretion pathways for dynamic and directed secretion of adhesive proteins during cell movement. Since newly synthesized FN is secreted from the Golgi as a soluble non-adhesive protein, it must be assembled into fibrils at the cell surface before it can support cell adhesion.16-18 Fibril assembly is likely to be a fairly slow process and may not occur at the leading edge of cells. By contrast, already assembled FN fibrils can be cleaved by extracellular proteinases, internalized, and resecreted in adhesive form from lysosomes to promote rapid adhesion assembly. Furthermore, lysosomal proteases themselves are known to generate FN fragments that are highly adhesive,19,20 which could further allow conversion of soluble internalized FN into an insoluble ready-to-adhere form. According to this model (Fig. 1), lysosomal secretion would be a rapid way to provide adhesive forms of FN and, if targeted to the leading edge, could alter the directionality or speed of migrating cells. One could also envision that targeted deposition of FN might reinforce a nascent protrusion to create a dominant lamellipodium and drive persistent cell migration.
Figure 1. A model of cell motility regulated by lysosomal secretion of ECM. In stationary cells, ECM is present in the environment in soluble non-adhesive form or as fibrils. For cells to transition to a motile state, they must adopt a new morphology that could be facilitated or stabilized by rapid secretion of adhesive forms of ECM. Thus, soluble ECM or proteolysed ECM fibrils could be internalized into lysosomes, cleaved into adhesive fragments by lysosomal enzymes, and resecreted at the basal cell surface adjacent to the lamellipodium to promote cell motility.
Although to our knowledge no previous group has studied the role of lysosomal secretion in ECM deposition, two prior studies have shown that lysosomal secretion significantly contributes to cell motility.21,22 Thus, inhibition of lysosomal secretion by expression of a dominant negative domain of the LE/Lys v-SNARE protein VAMP7 (vesicle-associated membrane protein-7) was accompanied by a reduction in the velocity of MDCK cells in a wound healing assay.21 In addition, using an shRNA screen Colvin et al. identified several lysosomal secretion regulators, including the secretory lysosome fusion regulator and VAMP7-interacting protein Syt7, as key regulators of leukocyte chemotaxis.22 Interestingly, in the 1970s Showell et al.23 identified a tight correlative relationship between induction of leukocyte chemotaxis and lysosome secretion by N-formyl methionyl peptides (correlation coefficient of 0.98 when comparing the ED50s of 19 different peptides in the two assays); the recent findings from ourselves and others suggest that this correlation may be mechanistically meaningful.
From recent work, lysosomal secretion appears to be used by a variety of cell types for cell motility and indeed other phenotypes,13,21,22,24-28 not just by hematopoietic cells that are known to contain specialized secretory granules of lysosomal origin. For example, membrane resealing in muscle cells and neurite outgrowth both depend on lysosomal secretion, suggesting that the classic description of lysosomes as waste disposal compartments may underestimate their versatility and utility.
In conclusion, we have described a novel mechanism by which the branched actin assembly protein cortactin promotes cell motility that involves lysosomal secretion of ECM. Many fascinating questions remain, including whether FN is the only motility molecule that is routed by lysosomal secretion, whether FN must be preassembled before endocytosis to promote effective cell motility, how cortactin specifically controls lysosomal secretion, and under what physiologic conditions lysosomal secretion is most decisive.
Acknowledgments
Funding support came from NIH 1R01GM075126 and ACS RSG -118085 grants. Thanks to Vito Quaranta for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/19197
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–8. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011;5:187–98. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Hamill KJ, Hopkinson SB, Jonkman MF, Jones JCR. Type XVII collagen regulates lamellipod stability, cell motility, and signaling to Rac1 by targeting bullous pemphigoid antigen 1e to α6β4 integrin. J Biol Chem. 2011;286:26768–80. doi: 10.1074/jbc.M110.203646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J Cell Biol. 2003;162:731–41. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res. 2005;302:83–95. doi: 10.1016/j.yexcr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–87. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayman EG, Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979;83:255–9. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayirhan-Denizli F, Arica MY, Denizli A. Fibronectin purification from human plasma in a packed-bed column system with gelatin immobilized PHEMA microspheres. J Biomater Sci Polym Ed. 2001;12:479–89. doi: 10.1163/156856201300194225. [DOI] [PubMed] [Google Scholar]
- 11.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 12.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, et al. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 2008;22:1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 13.Sung BH, Zhu X, Kaverina I, Weaver AM. Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Curr Biol. 2011;21:1460–9. doi: 10.1016/j.cub.2011.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill KJ, Kligys K, Hopkinson SB, Jones JCR. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci. 2009;122:4409–17. doi: 10.1242/jcs.041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–63. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol. 1999;11:622–7. doi: 10.1016/S0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 17.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3:a005041.. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphries MJ, Ayad SR. Stimulation of DNA synthesis by cathepsin D digests of fibronectin. Nature. 1983;305:811–3. doi: 10.1038/305811a0. [DOI] [PubMed] [Google Scholar]
- 20.Fukai F, Suzuki H, Suzuki K, Tsugita A, Katayama T. Rat plasma fibronectin contains two distinct chemotactic domains for fibroblastic cells. J Biol Chem. 1991;266:8807–13. [PubMed] [Google Scholar]
- 21.Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol Cell. 2007;99:261–71. doi: 10.1042/BC20060097. [DOI] [PubMed] [Google Scholar]
- 22.Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. 2010;11:495–502. doi: 10.1038/ni.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Showell HJ, Freer RJ, Zigmond SH, Schiffmann E, Aswanikumar S, Corcoran B, et al. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976;143:1154–69. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol. 2008;18:926–31. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Arantes RME, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–7. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–9. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu C, Ortega-Cava CF, Chen G, Fernandes ND, Cavallo-Medved D, Sloane BF, et al. Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res. 2008;68:9147–56. doi: 10.1158/0008-5472.CAN-07-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H, Ito Y, Chappel J, Andrews NW, Teitelbaum SL, Ross FP. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev Cell. 2008;14:914–25. doi: 10.1016/j.devcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]